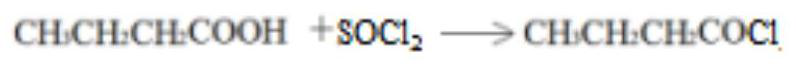Method for synthesizing functional food antioxidant dibutyrin ferulate
A technology of glyceryl dibutyrate and functional food, which is applied in the field of preparation of food antioxidants, can solve the problems of many by-products, mixing, and difficulty in purification, and achieve the effect of mild preparation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The method for functional food antioxidant ferulic acid bisbutyrin, comprises the steps:
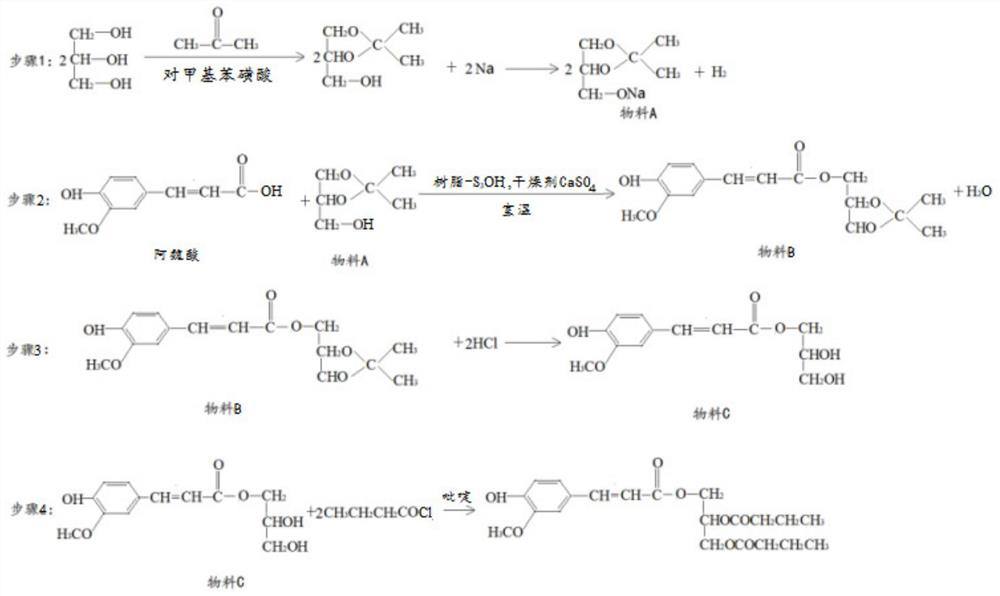
[0028] Step 1, add acetone, glycerol chloroform and catalyst sodium p-toluenesulfonate in the dry there-necked flask equipped with electric stirrer and Soxhlet extractor, anhydrous Na is housed in the extraction cylinder in the Soxhlet extractor 2 SO 4 , after heating to reflux for 4.5h, add NaHCO 3 To pH=7, filter while hot, carry out vacuum distillation at 100°C and 30mmHg, collect fractions, and freeze-dry to obtain isopropylidene glycerol, namely material A;
[0029] Step 2, mixing ferulic acid with the material A prepared in step 1, adding a strong acidic cation exchange resin, and stirring at room temperature for 12 hours to obtain material B;
[0030] Step 3, adding dropwise a dilute hydrochloric acid solution with a concentration of 0.5 mol / L to the material B prepared in step 3, heating to reflux, adjusting the pH to neutral, washing with water, and obtaining material C...
Embodiment 2
[0033] The method for functional food antioxidant ferulic acid bisbutyrin, comprises the steps:
[0034] Step 1, add acetone, glycerol chloroform and catalyst sodium p-toluenesulfonate in the dry there-necked flask equipped with electric stirrer and Soxhlet extractor, anhydrous Na is housed in the extraction cylinder in the Soxhlet extractor 2 SO 4 , after heating to reflux for 5h, add NaHCO 3 To pH=7, filter while hot, carry out vacuum distillation at 100°C and 30mmHg, collect fractions, and freeze-dry to obtain isopropylidene glycerol, namely material A;
[0035] Step 2, mixing ferulic acid with the material A prepared in step 1, adding a strong acidic cation exchange resin, and stirring at room temperature for 10 hours to obtain material B;
[0036] Step 3, adding dropwise a dilute sulfuric acid solution with a concentration of 0.75 mol / L to the material B prepared in step 3, heating to reflux, adjusting the pH to neutral, washing with water, and obtaining material C;
...
Embodiment 3
[0039] The method for functional food antioxidant ferulic acid bisbutyrin, comprises the steps:
[0040] Step 1, add acetone, glycerol chloroform and catalyst sodium p-toluenesulfonate in the dry there-necked flask equipped with electric stirrer and Soxhlet extractor, anhydrous Na is housed in the extraction cylinder in the Soxhlet extractor 2 SO 4 , after heating to reflux for 5.5h, add NaHCO 3 To pH=7, filter while hot, carry out vacuum distillation at 100°C and 30mmHg, collect fractions, and freeze-dry to obtain isopropylidene glycerol, namely material A;
[0041] Step 2, mixing ferulic acid with the material A prepared in step 1, adding a strong acidic cation exchange resin, and stirring at room temperature for 8 hours to obtain material B;
[0042] Step 3, adding dropwise a dilute hydrochloric acid solution with a concentration of 0.5 mol / L to the material B prepared in step 3, heating to reflux, adjusting the pH to neutral, washing with water, and obtaining material C;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com