Preparation method of elexacaftor intermediate and application thereof
A technology of intermediates and reactions, applied in the field of synthesis of pharmaceutical intermediates, can solve problems such as unfavorable scale-up production and harsh reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
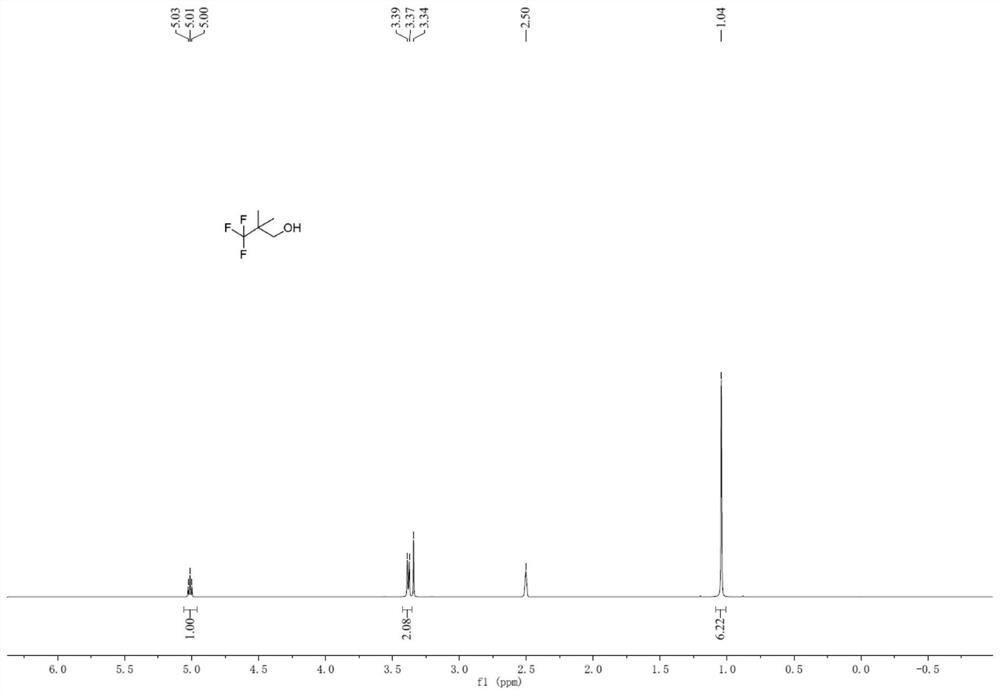
[0084] The present invention also provides a method for preparing 3,3,3-trifluoro-2,2-dimethylpropan-1-ol, the method comprising the following steps:
[0085] (1) Add 3,3,3-trifluoropropionic acid and protective reagent to the solvent, continue to add condensation reagent to obtain intermediate A, the reaction equation is as follows:
[0086]
[0087] Wherein, G is selected from -OR 3 and-NR 4 R 5 ,in,
[0088] R 3 selected from substituted or unsubstituted C 1-6 Alkyl, C 3-7 Cycloalkyl, 3-7 membered heterocyclyl, C 6-10 Aryl and 5-10 membered heteroaryl;
[0089] R 4 and R 5 are independently selected from substituted or unsubstituted C 1-6 Alkyl, C 3-7 Cycloalkyl, 3-7 membered heterocyclyl, C 6-10 Aryl and 5-10 membered heteroaryl, or R 4 and R 5 Linked to form a substituted or unsubstituted 3-7 membered heterocyclic group;
[0090] (2) The above-mentioned intermediate A, methylating reagent and alkali are added in the solvent, and the reaction obtains the ...
Embodiment 1
[0109] Add 3,3,3-trifluoropropionic acid (500.0g), dichloromethane (5L), cyclohexanol (585g), and DMAP (95.3g) into the reaction flask, stir and dissolve, then cool down to -5~15°C, Add DCC (844g) in batches to it, and rise to room temperature to react for 3h, the raw material is completely converted, then add 1N hydrochloric acid aqueous solution (0.8L), 5% sodium bicarbonate aqueous solution (1L), and saturated brine (500mL) to wash , the organic phase was dried and concentrated to obtain 857g of crude product, which was distilled under reduced pressure to obtain 803g of 3,3,3,-cyclohexyl trifluoropropionate, with a molar yield of 98%.
[0110] Add 3,3,3,-cyclohexyl trifluoropropionate (150.0g), acetone (900mL), potassium carbonate (295g) and methyl iodide (253g) into a 2L three-necked flask, stir well and heat up to reflux for 6h. The reaction solution was filtered, the filter cake was rinsed with a small amount of acetone, the filtrates were combined, and purified by disti...
Embodiment 2
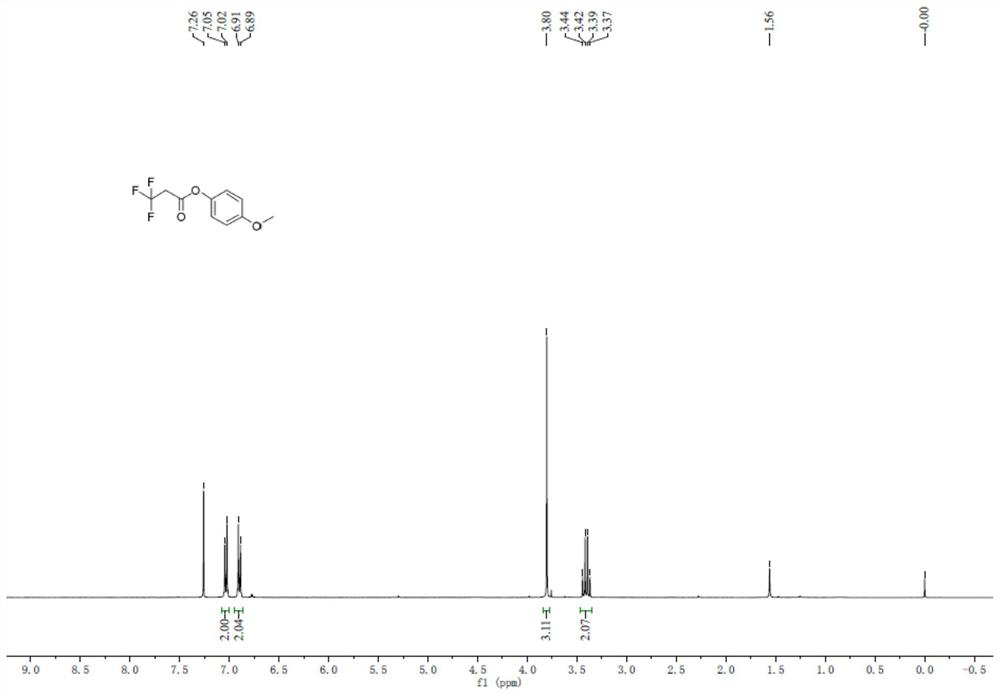
[0113] Add 3,3,3-trifluoropropionic acid (50.0g), tetrahydrofuran (500mL), 4-methoxyphenol (48.5g), DMAP (9.5g) into the reaction flask, stir to dissolve and cool down to -5~15 ℃, added EDCI (36g) in batches, raised to room temperature and reacted for 3h, the raw material was completely converted, then added 1N aqueous hydrochloric acid (0.2L), washed with 5% aqueous sodium bicarbonate (0.2L), and dried the organic phase , concentrated, and purified by distillation under reduced pressure to obtain 88 g of 3,3,3,-p-methoxyphenyl trifluoropropionate, with a molar yield of 97%.
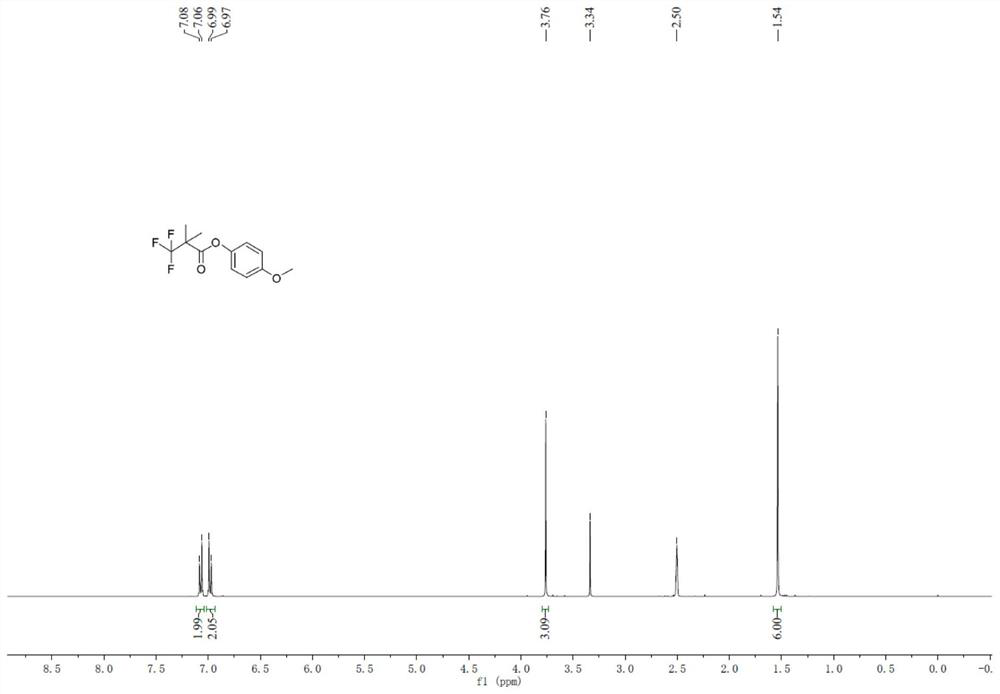
[0114] Add 3,3,3,-p-methoxyphenyl trifluoropropionate (30.0g), acetonitrile (200mL), cesium carbonate (84g), dimethyl sulfate (48.4g) into the reaction flask, stir well and then heat up After reflux for 4 hours, the reaction liquid was filtered, and the filtrate was purified by distillation to obtain 31.2 g of 3,3,3,-trifluoropropionic acid-2,2-dimethyl-p-methoxyphenyl ester, with a molar yield of 92%. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com