Long-acting injectable formulations and crystalline forms of buprenorphine derivatives
A crystalline, formalized technology for the treatment of opioid dependence, pain, and depression that addresses problems without significant burst effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Example 1: Synthesis of 3-acyl-buprenorphine derivatives
[0081] Described below is the method used to synthesize 3-acyl-buprenorphine derivatives. Add the suspension of buprenorphine hydrochloride and dichloromethane (DCM) to a suitable three-neck round bottom flask and place in an ice bath to cool. Then, trimethylamine (TEA) was slowly added under stirring, and acid chloride was added dropwise into the flask. After all materials were added, the ice bath was stopped. The reaction mixture was reacted at ambient temperature for 1 to 4 hours, and the reaction mixture was neutralized with saturated aqueous sodium bicarbonate solution. The organic layer was washed with brine, then dried over sodium sulfate. After condensation under reduced pressure, crude buprenorphine derivatives were obtained (Table 1).
[0082] Table 1. Synthetic conditions for various 3-acyl-buprenorphine derivatives
[0083]
Embodiment 2
[0084] Example 2: Crystallization of 3-acyl-buprenorphine derivatives
[0085] The following is the crystallization method of 3-acyl-buprenorphine derivatives. The crude 3-acyl-buprenorphine derivatives were dissolved in the solvents described in Table 2 at ambient temperature or in a heated oil or water bath. Then, the dissolved mixture was cooled in an ice bath to form a crystalline 3-acyl-buprenorphine derivative.
[0086] Table 2. Crystallization conditions for 3-acyl-buprenorphine derivatives
[0087]
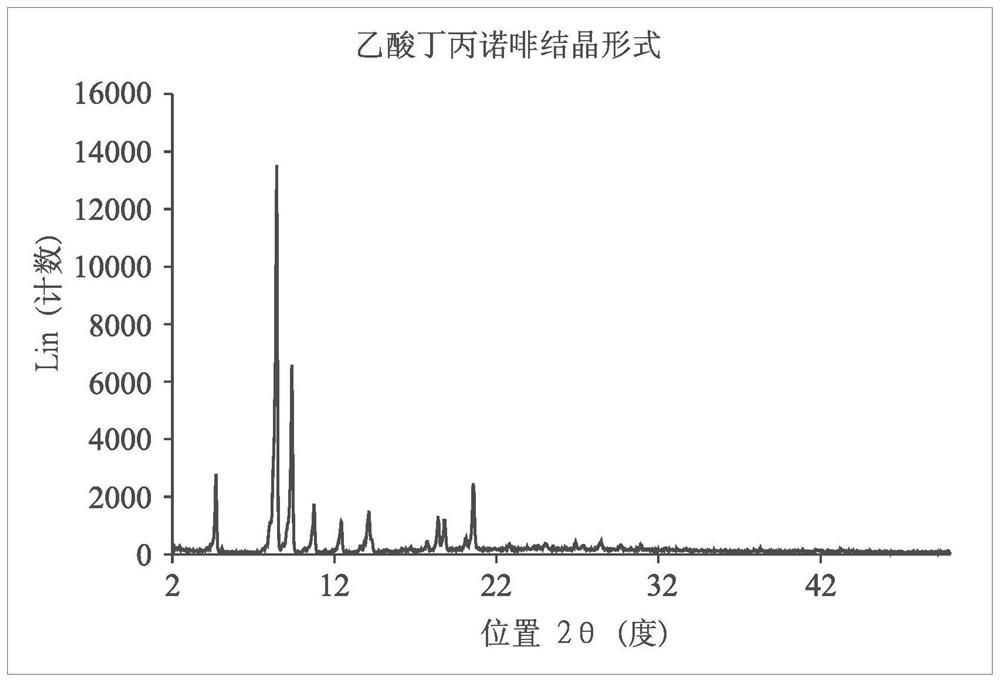
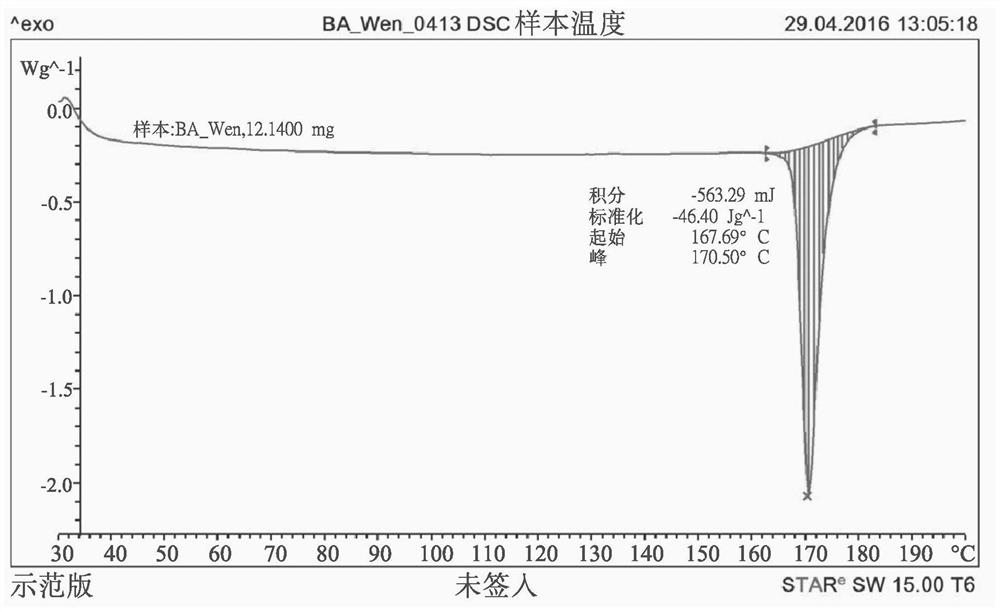
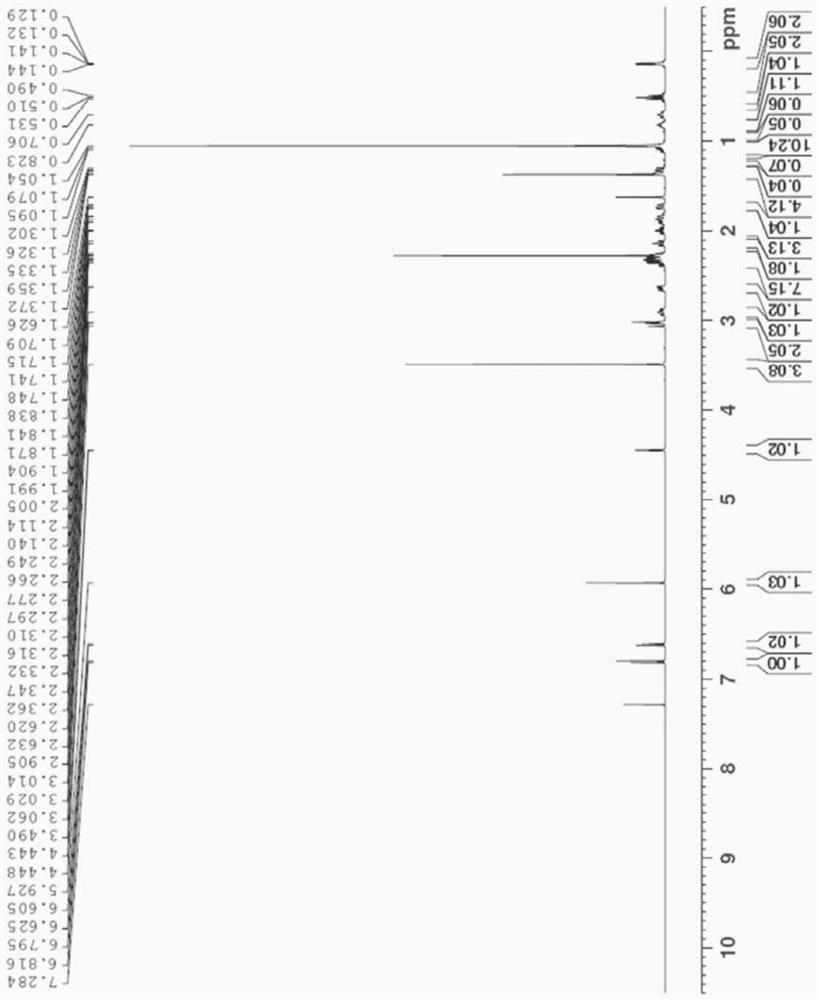
[0088] The crystalline 3-acyl-buprenorphine derivative obtained above was characterized by XRD, DSC, NMR and IR.
[0089] The crystalline form of buprenorphine acetate is characterized by an X-ray diffraction pattern (Bruker, D8 DISCOVER SSS multipurpose thin-film X-ray diffractometer), with peaks at 4.70, 8.44, 9.38, 10.74, 12.42, 14.12 , 17.72, 18.40, 18.78, 20.08, 20.56, 25.04, 26.88, 28.42, 28.46 degrees 2θ ( figure 1 ), and its melting point was determined to b...
Embodiment 3
[0097] Example 3: Solubility of buprenorphine derivatives and their salt forms in dissolution media
[0098] An excess of compound (including buprenorphine free base, buprenorphine derivative or its salt form) was weighed into a glass tube containing 5 mL of dissolution medium. The medium is the same as the previous embodiment. The tube was then sealed and placed in a reciprocating shaker at 60 rpm in a 55°C water bath. The solution was filtered through a 0.45 μm nylon filter, and then the filtrate was further diluted with acetonitrile, and the content of each compound was determined by HPLC.
[0099] Table 3. Solubility of Buprenorphine Derivatives
[0100]
[0101] Table 4. Solubility of buprenorphine derivative salt forms
[0102]
[0103]
[0104] Table 3 shows that the buprenorphine derivative (crystalline form) is less soluble than its parent compound (buprenorphine free base). The crystalline form of buprenorphine decanoate is nearly 10 times less soluble t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com