Application of Abemaciclib in preparation of medicine for treating NAFLD
A drug, liver technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
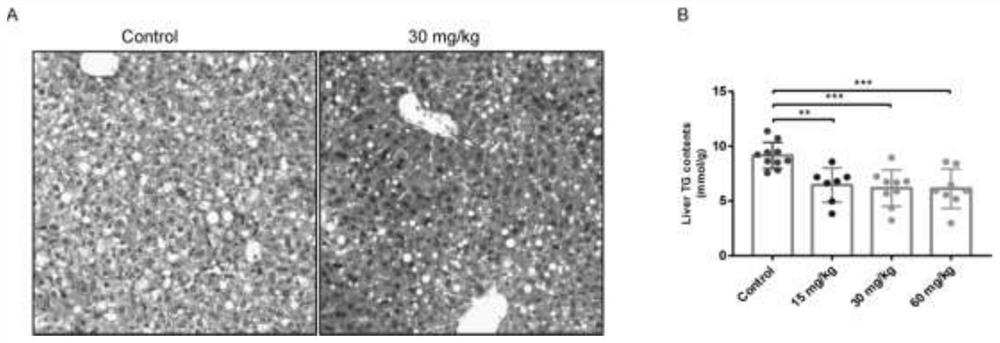
[0020] Example 1, Abemaciclib reduces hepatic steatosis and reduces triglyceride content in the liver
[0021] 50 C57BL / 6 mice, male, 6-8 weeks old, were randomly divided into five groups according to body weight after environmental adaptation, with 10 mice in each group, namely control group, Abemaciclib low dose (15mg / Kg), middle dose (30mg / Kg) Kg), high dose (60mg / Kg) group. The mice in each group were given MCD feed to construct the NASH model. Except the control group was given the corresponding volume of animal drinking water, the other groups were given the corresponding drug intervention for 3 consecutive weeks. At the end of the administration, the liver was dissected and removed, and part of the liver lobe was cut out and fixed in formalin solution for HE staining to detect the lipid degeneration of the liver tissue. The degree of fatty degeneration of liver tissue in both groups was significantly reduced ( figure 1 A). In addition, part of the liver lobe was cut ...
Embodiment 2
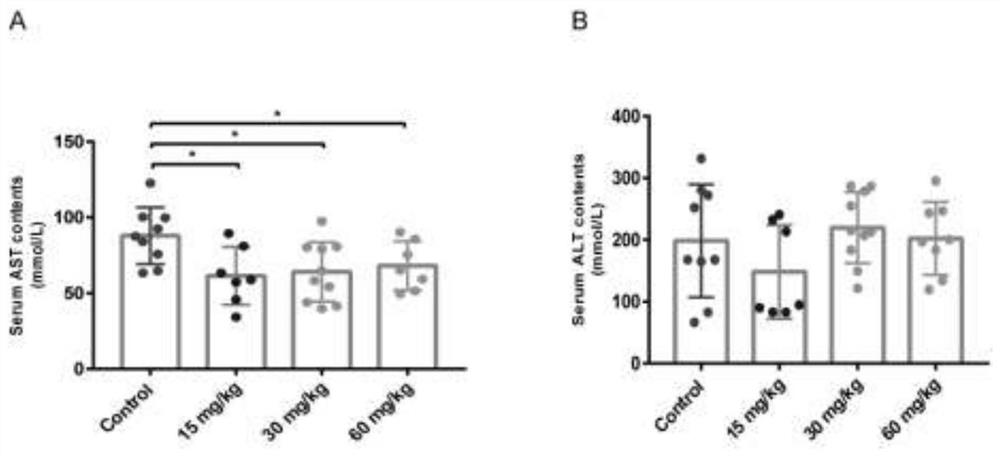
[0022] Example 2, Abemaciclib improves abnormal liver function caused by hepatic steatosis
[0023] NASH model construction and intervention measures are the same as figure 1 After the administration of animals in each group, the blood was collected from the orbit, and the serum was collected by centrifugation at 3000rpm for 10min at 4°C. The contents of ALT and AST in the serum were detected according to the kit instructions. The results showed that compared with the control group, 15mg / kg, 30mg / kg, 60mg / kg The serum AST in the kg dose group was significantly lower than that in the control group, suggesting that Abemaciclib can improve liver function abnormalities caused by hepatic steatosis ( figure 2 A-B).
Embodiment 3
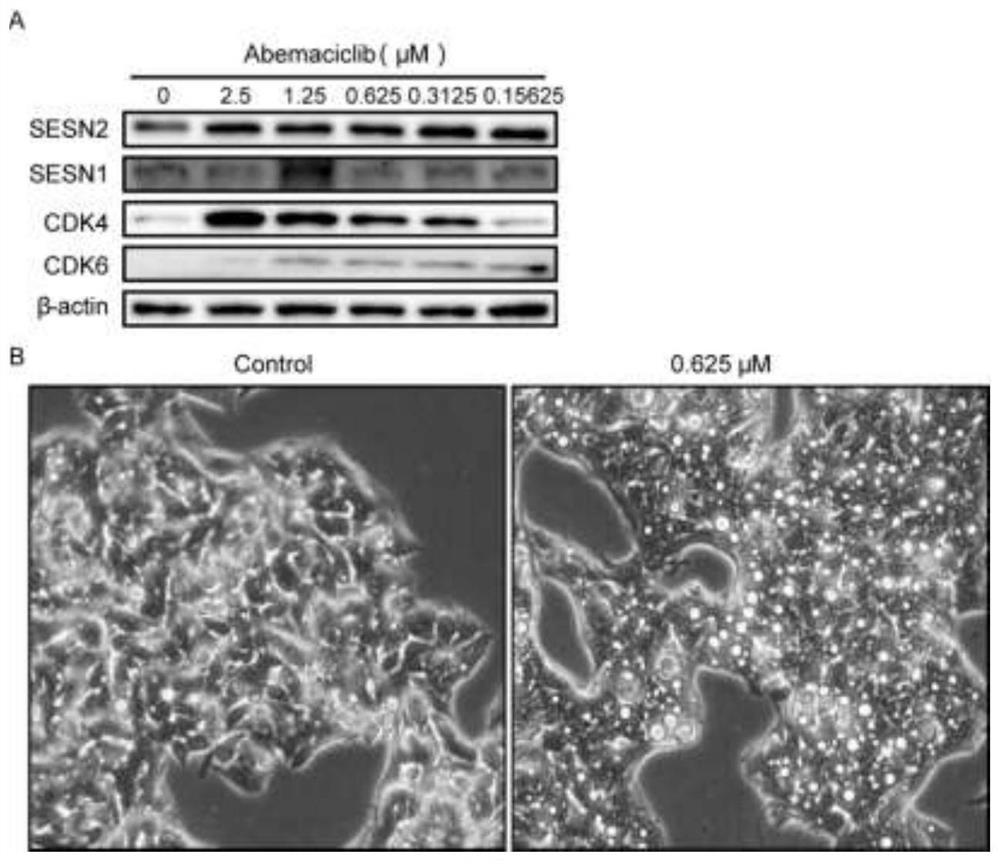
[0024] Example 3, Abemaciclib upregulates SESN2 to induce autophagy and improves NAFLD
[0025] HepG2 cells were inoculated into two 6-well plates one day in advance, and Abemaciclib was prepared according to the concentration gradient of 2.5 μM, 1.25 μM, 0.625 μM, 0.3125 μM, and 0.15625 μM the next day. After administration, the cells were placed in a 37°C, 5% CO2 incubator to continue culturing for 24 hours. One of the 6-well plates was used to extract the total protein and perform Western blot to detect the expression of related proteins. The other 6-well plate was placed under a microscope for observation and Photograph. The results showed that compared with the control group, the expression of the antioxidant protein SESN2 was significantly up-regulated in a dose-dependent manner when different concentrations of Abemaciclib were administered to HepG2 cells for 48 hours, while SESN1 had no significant change. At the same time, our experimental results showed that the expr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com