Fluorescent sensing film as well as preparation method and application thereof
A fluorescent sensing and thin film technology, applied in the field of fluorescent sensing thin film and its preparation, can solve the problems of low detection efficiency, high detection environment requirements, complex operation, etc., achieve controllable and uniform thickness, improve film forming effect, surface uniform effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
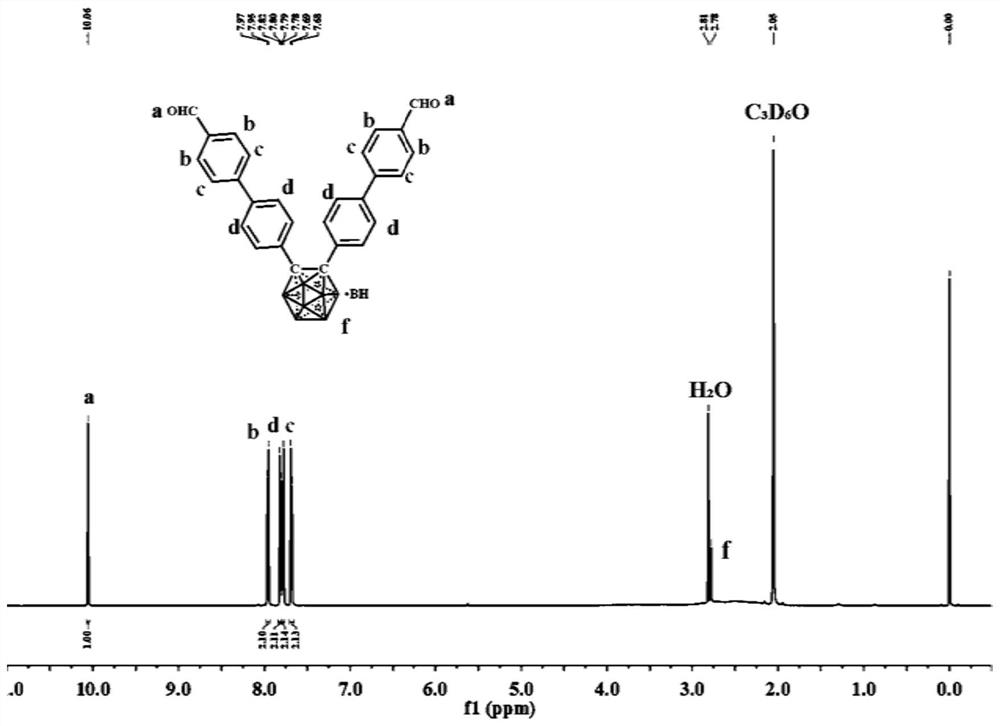
[0065] The present invention also discloses a synthesis method of the above-mentioned trimesisamide and o-carborane derivatives, comprising the following steps:
[0066] 1) Preparation of compound 1
[0067] Add 1,3,5-benzenetricarboxylic acid chloride, methyl p-aminobenzoate, triethylamine and tetrahydrofuran into the flask, and under the protection of an inert atmosphere, stir at room temperature for 9 to 12 hours. After the reaction is completed, filter to remove the precipitate. The solvent was concentrated under reduced pressure, and then methanol was added, and a white precipitate appeared immediately, the solution was filtered with suction, and the filter cake was washed with water and methanol several times, and the obtained product was dried to obtain compound 1;
[0068] Wherein, the structural formula of the compound 1 is:
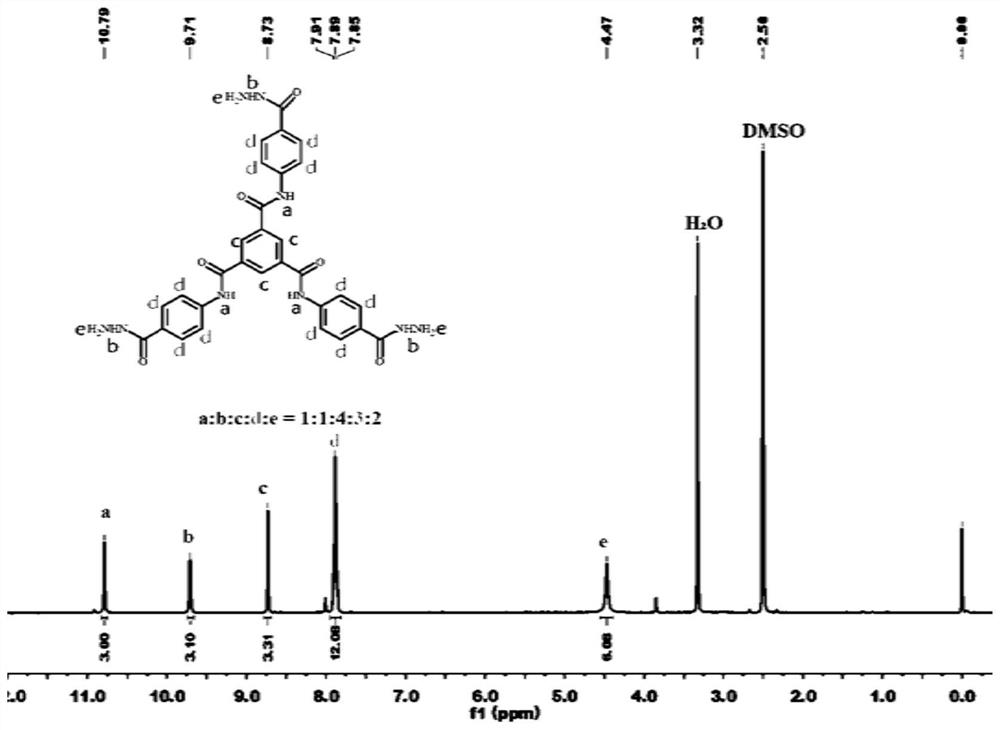
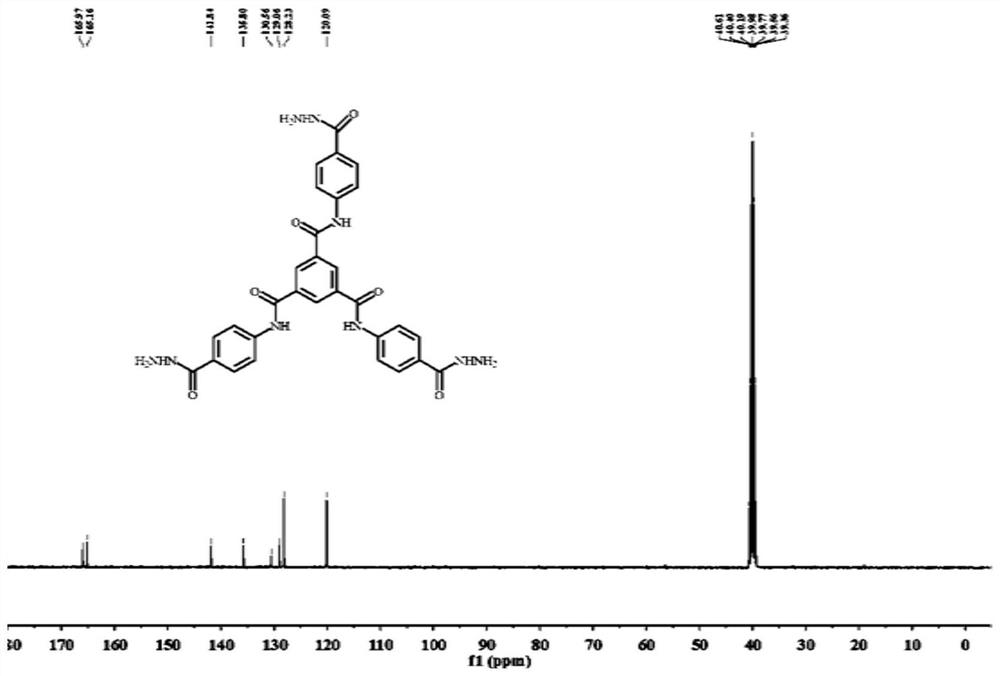
[0069] 2) Preparation of Compound 2 (Trimellitic Tricarboxamides)
[0070] Add compound 1, methanol, and hydrazine hydrate into the flask i...
Embodiment 1
[0095] 1.1 Preparation of target compounds of trimesamide and o-carborane derivatives
[0096] 1) Synthesis of compound 1
[0097] Add 0.26g of 1,3,5-benzenetricarboxylic acid chloride, 0.76g of methyl p-aminobenzoate, 1ml of triethylamine and 30mL of tetrahydrofuran into the flask in sequence, and react under reflux at 20°C for 10 hours under a nitrogen atmosphere. After the reaction is completed, , the reaction solution was concentrated under reduced pressure, and 100 mL of methanol was added to the concentrated solution, and a white precipitate appeared immediately, the solution was filtered with suction, and the filter cake was washed with water and methanol several times, and the obtained substance was vacuum-dried to obtain a white compound 1;
[0098] Its reaction equation is as follows:
[0099]
[0100] 2) Synthesis of compound 2 (trimellitic tricarboxamides)
[0101]Add 0.2g of compound 1, 5mL of hydrazine hydrate, and 20mL of methanol into a 100mL two-necked ro...
Embodiment 2
[0116] 2.1 Preparation of target compounds of trimesamide and o-carborane derivatives
[0117] 1) Synthesis of compound 1
[0118] Add 0.26g of 1,3,5-benzenetricarboxylic acid chloride, 1.06g of methyl p-aminobenzoate, 2ml of triethylamine and 35mL of tetrahydrofuran into the flask in sequence, and react under reflux at 25°C for 11 hours under a nitrogen atmosphere. After the reaction is completed, , the reaction solution was concentrated under reduced pressure, and 100 mL of methanol was added to the concentrated solution, and a white precipitate appeared immediately, the solution was filtered with suction, and the filter cake was washed with water and methanol several times, and the obtained substance was vacuum-dried to obtain a white compound 1;
[0119] 2) Synthesis of compound 2 (trimellitic tricarboxamides)
[0120] Add 0.2g of compound 1, 8mL of hydrazine hydrate, and 30mL of methanol into a 100mL two-necked round-bottomed flask in sequence, and stir under reflux at 7...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com