Cationic cyclodextrin-based hydrogel adsorbing material and synthetic method thereof
A technology of adsorption materials and synthesis methods, applied in chemical instruments and methods, adsorption water/sewage treatment, water pollutants, etc., can solve the problems of ineffectiveness, small adsorption amount, and less research, and achieve easy access and good quality Recyclability, the effect of simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
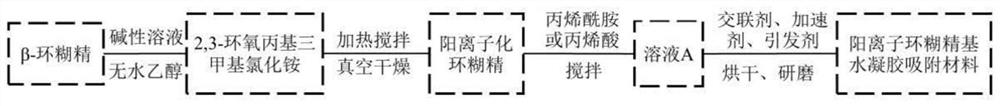
[0035] Dissolve 1g of β-cyclodextrin in 25ml of 0.8wt.% sodium hydroxide solution, stir until completely mixed, add 6.5g of 2,3-epoxypropyltrimethylammonium chloride to the solution, heat at 70°C Stir for 4 hours; vacuum dry to constant weight at 80°C to obtain β-cyclodextrin cationic derivative.
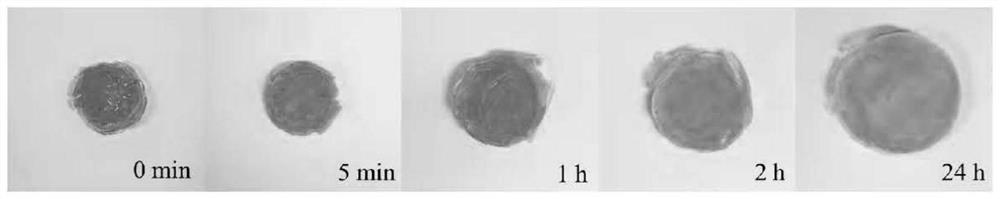
[0036] The obtained β-cyclodextrin cationic derivative was dissolved in 2.5 ml of water, 2.5 g of acrylamide was added, and the mixture was stirred at room temperature for 1 hour at a rotational speed of 500 rpm. Then add 0.2g cross-linking agent N,N'-methylenebisacrylamide (MBA), stir until the cross-linking agent MBA is completely dissolved, add 0.25mL accelerator N,N,N',N'-tetramethyl ethyl acetate Diamine (TEMED). When the above substances are mixed uniformly, add 5 mL of saturated potassium persulfate solution (KPS) solution of initiator, and stir at room temperature until hydrogel is formed. The resulting hydrogel was transferred to a glass bottle and immersed in deionized w...
Embodiment 2
[0039] Dissolve 1.5g of β-cyclodextrin in 25ml of 0.8wt.% sodium hydroxide solution, stir until completely mixed, add 10.5g of 2,3-epoxypropyltrimethylammonium chloride to the solution, heat for 70 Stir at °C for 4 hours; vacuum dry at 80 °C to constant weight to obtain β-cyclodextrin cationic derivative.
[0040] The obtained β-cyclodextrin cationic derivative was dissolved in 2.5 ml of water, 2.5 g of acrylic acid was added, and the mixture was stirred at room temperature for 1 hour at a rotational speed of 500 rpm. Then add 0.2g cross-linking agent N,N'-methylenebisacrylamide (MBA), stir until the cross-linking agent MBA is completely dissolved, add 0.25mL accelerator N,N,N',N'-tetramethyl ethyl acetate Diamine (TEMED). When the above substances are mixed evenly, add 5 mL of saturated ammonium persulfate solution (APS) solution of the initiator, and stir at room temperature until a hydrogel is formed. The resulting hydrogel was transferred to a glass bottle and immersed i...
Embodiment 3
[0043] Dissolve 1g of β-cyclodextrin in 25ml of 8wt.% sodium hydroxide solution, stir until completely mixed, add 10.5g of 2,3-epoxypropyltrimethylammonium chloride to the solution, heat at 70°C and stir 6 hours; vacuum drying at 80°C to constant weight to obtain β-cyclodextrin cationic derivatives.
[0044] The obtained β-cyclodextrin cationic derivative was dissolved in 2.5 ml of water, 1 g of acrylic acid was added, and the mixture was stirred at room temperature for 1 hour at a rotational speed of 500 rpm. Then add 0.2g cross-linking agent N,N'-methylenebisacrylamide (MBA), stir until the cross-linking agent MBA is completely dissolved, add 0.25mL accelerator N,N,N',N'-tetramethyl ethyl acetate Diamine (TEMED). When the above substances are mixed uniformly, add 5 mL of saturated potassium persulfate solution (KPS) solution of initiator, and stir at room temperature until hydrogel is formed. The resulting hydrogel was transferred to a glass bottle and immersed in deionize...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum adsorption capacity | aaaaa | aaaaa |
| Maximum adsorption capacity | aaaaa | aaaaa |
| Maximum adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com