Photocatalytic nano composite catalyst and preparation method thereof
A nanocomposite and catalyst technology, applied in the field of photocatalytic nanomaterial synthesis, can solve the problems of low photocatalytic pure water splitting efficiency, high price, scarce reserves of precious metal catalysts, etc., and achieve good catalytic activity and stability, low price, The effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A preparation method of a photocatalytic nanocomposite catalyst, comprising the following steps:
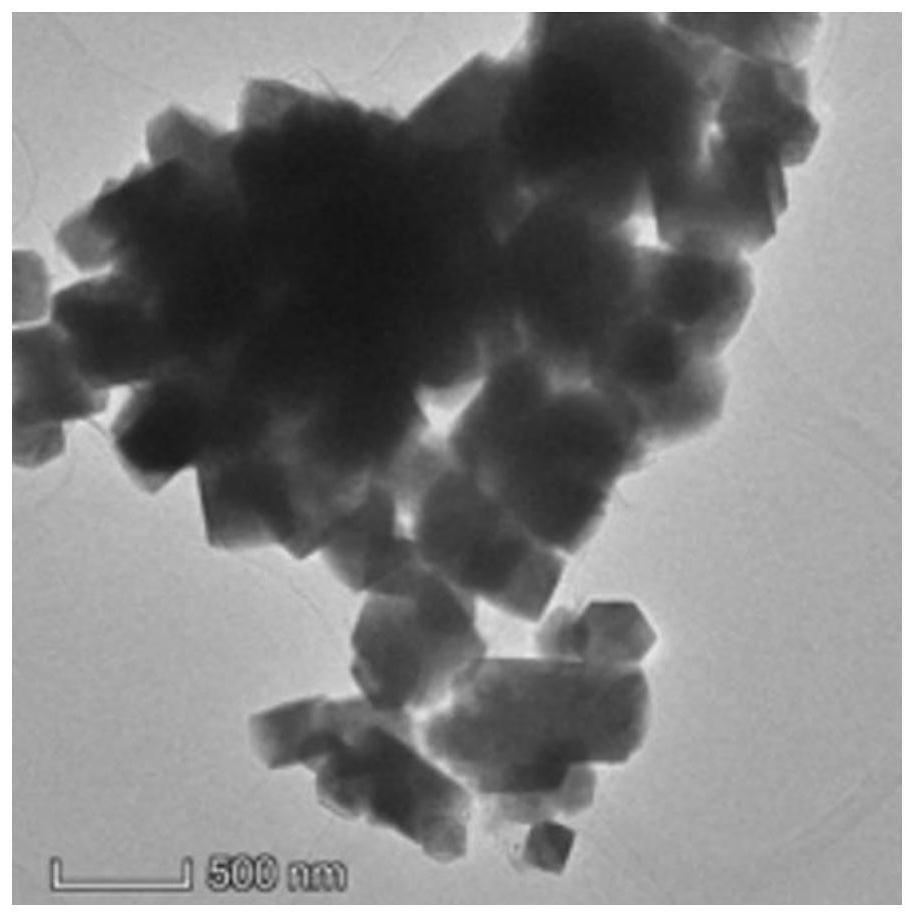
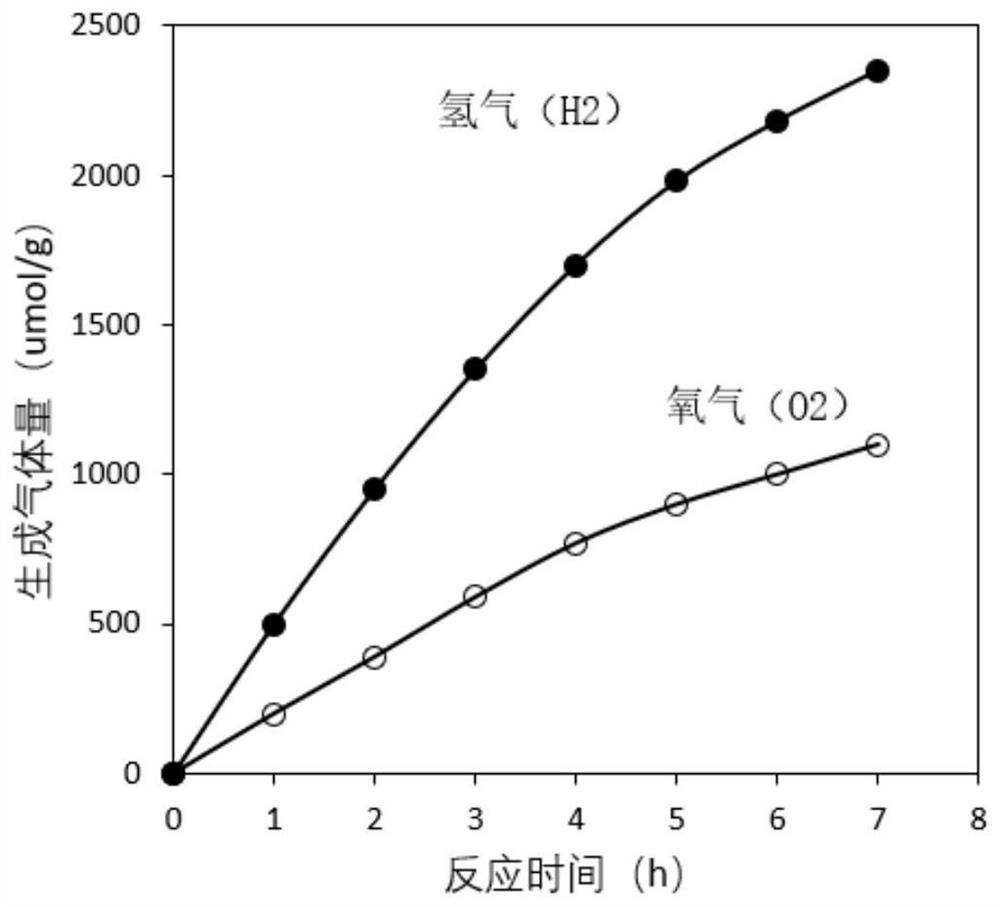
[0026] Weigh 0.008mol of lanthanum nitrate, 0.008mol of aluminum nitrate and 0.084mol of SrTiO, respectively 3 , mixed and ground in an agate mortar for 1 hour, the ground mixture was placed in a ball mill, ball milled at 4000 revolutions for 10 hours, and then 1 mol of SrCl was added. 2 Continue ball milling for 1 hour; sieve and separate the ball-milled mixture, place it in a muffle furnace, keep it at 800 degrees for 12 hours, take it out, filter and wash it with a large amount of deionized water, and then place the material in a drying box at 80 degrees Dry overnight to obtain La,Al-SrTiO 3 Nanoparticles; La,Al-SrTiO 3 With the mass ratio of 2.0wt% (NH 4 ) 2 WS 4 WS formed after 800 degree pyrolysis peeling 2The nanosheets and chromium salts were uniformly ground and mixed for 4 hours, and kept at 500 degrees for 4 hours under the protection of inert gas to obtai...
Embodiment 2
[0029] A preparation method of a photocatalytic nanocomposite catalyst, comprising the following steps:
[0030] Weigh 0.005mol of lanthanum nitrate, 0.005mol of aluminum nitrate and 0.09mol of SrTiO respectively 3 , mixed and ground in an agate mortar for 1 hour, the ground mixture was placed in a ball mill, ball-milled at 4000 revolutions for 24 hours, and then 1 mol of SrCl was added. 2 Continue ball milling for 1 hour; sieve and separate the ball-milled mixture, place it in a muffle furnace, and keep it at 1000 degrees for 8 hours; after taking it out, filter and wash it with a large amount of deionized water, and then place the material in a drying box at 80 degrees Dry overnight to obtain La,Al-SrTiO 3 Nano catalyst material; La,Al-SrTiO 3 With the mass ratio of 1.0wt% (NH 4 ) 2 WS 4 WS formed after 400 degree pyrolysis peeling 2 The nanosheets and the chromium salt were uniformly ground and mixed for 4 hours, and kept at 400 degrees for 2 hours under the protectio...
Embodiment 3
[0032] A preparation method of a photocatalytic nanocomposite catalyst, comprising the following steps:
[0033] Weigh 0.001mol of lanthanum nitrate, 0.001mol of aluminum nitrate and 0.098mol of SrTiO respectively 3 , mixed and ground in an agate mortar for 1 hour, the ground mixture was placed in a ball mill, ball-milled at 4000 revolutions for 24 hours, and then 1 mol of SrCl was added. 2 Continue ball milling for 1 hour; sieve and separate the ball-milled mixture, place it in a muffle furnace, and keep it at 800 degrees for 4 hours; after taking it out, filter and wash it with a large amount of deionized water, and then place the material in a drying box at 80 degrees Dry overnight to obtain La,Al-SrTiO 3 Nano catalyst material; La,Al-SrTiO 3 with a mass ratio of 0.5wt% (NH 4 ) 2 WS 4 WS formed after 400 degree pyrolysis peeling 2 The nanosheets and chromium salts were uniformly ground and mixed for 4 hours, and kept at 500 degrees for 4 hours under the protection of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com