Synthesis method of 2-acetylfuran
A technology for the synthesis of acetylfuran and its synthesis method, which is applied in the field of chemical synthesis of 2-acetylfuran, can solve the problems of high price, difficult separation, difficult acetic acidification reagents, etc., and achieve less solid waste, clean production environment, and low reaction cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
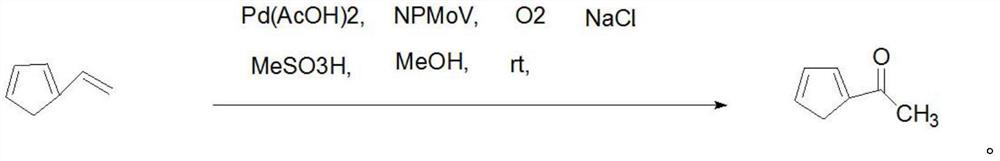
[0026] First add MeOH-water solution (100:1, v / v, 300ml) into the reactor, then add Pd(OAc) 2 (4.5mg, 0.02mmol), NPMoV[(NH 4 ) 5 h 6 PMo 4 V 7.8 o 40 .nH 2 O] (39mg, 0.025mmol), NaCl (2.93mg, 0.05mmol) and MeSO 3 H (19.2mg, 0.2mmol), stir well. Oxygen was then passed into the reactor, and then 2-vinylfuran (188mg, 2mmol) in MeOH-water solution ((100:1, v / v, 150ml)) was added dropwise to the catalytic system within 3 hours. After stirring at room temperature for 30 minutes, the solvent was distilled off under reduced pressure, and the obtained residue was separated by column chromatography (the chromatographic column was UltimateXB-C18 column (250mm*4.6mm, 5μm), and the mobile phase was methanol:0.5% formic acid=60:40 ), to obtain the target product 2-acetylfuran (184.24mg, yield 98.0%), liquid phase purity 99.85%.
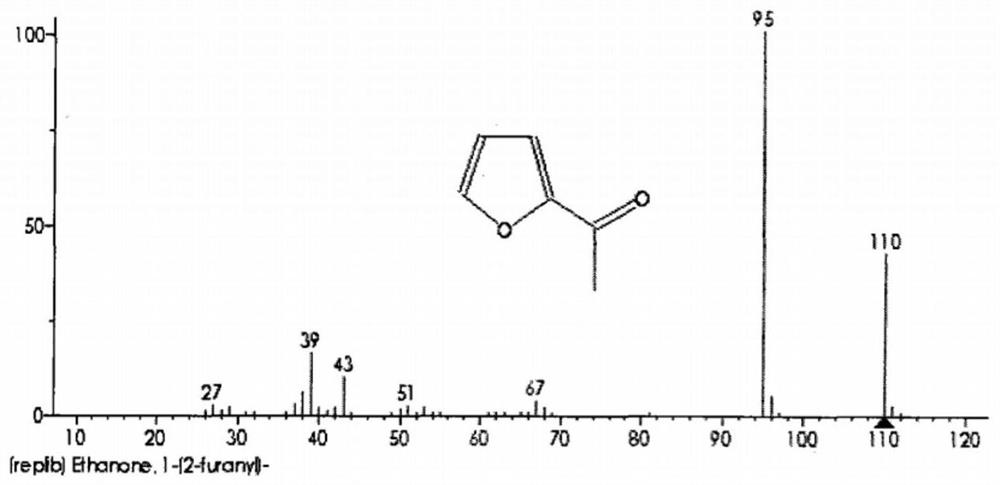
[0027] The mass spectrum of 2-acetylfuran (using Agilent Technologies 5973Network mass spectrometer, EI, 70ev): m / e: 110 (M + ),95(M-CH 3 ),67(M-COCH 3...
Embodiment 2
[0029] First add MeOH-water solution (80:1, v / v, 200ml) into the reactor, then add Pd(OAc) 2 (9.0mg, 0.04mmol), NPMoV[(NH 4 ) 5 h 6 PMo 4 V 7.8 o 40 .nH 2 O] (42mg, 0.027mmol), NaCl (1.47mg, 0.025mmol) and MeSO 3 H (9.6mg, 0.1mmol), stir well. Oxygen was then passed into the reactor, and then 2-vinylfuran (188g, 2mmol) in MeOH-water solution ((80:1, v / v, 100ml)) was added dropwise to the catalytic system within 3 hours. After stirring at room temperature for 30 minutes, the solvent was distilled off under reduced pressure, and the obtained residue was separated by column chromatography (the chromatographic column was UltimateXB-C18 column (250mm*4.6mm, 5μm), and the mobile phase was methanol:0.5% formic acid=60:40 ), the target product 2-acetylfuran (184.62g, yield 98.2%) was obtained, and the liquid phase purity was 99.7%.
Embodiment 3
[0031] First add MeOH-water solution (60:1, v / v, 160ml) into the reactor, then add Pd(OAc) 2 (13.5mg, 0.06mmol), NPMoV[(NH 4 ) 5 h 6 PMo 4 V 7.8 o 40 .nH 2 O] (45mg, 0.029mmol), NaCl (2.34mg, 0.04mmol) and MeSO 3 H (4.8mg, 0.05mmol), stir well. Oxygen was then passed into the reactor, and then 2-vinylfuran (188g, 2mmol) in MeOH-water solution ((60:1, v / v, 80ml)) was added dropwise to the catalytic system within 3 hours. After stirring at room temperature for 30 minutes, the solvent was distilled off under reduced pressure, and the obtained residue was separated by column chromatography (the chromatographic column was Ultimate XB-C18 column (250mm*4.6mm, 5μm), and the mobile phase was methanol: 0.5% formic acid=60: 40), the target product 2-acetylfuran (184.43g, yield 98.1%) was obtained, and the product liquid phase purity was 99.8%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com