PD-L1 targeting polypeptide as well as preparation method and application thereof
A PD-L1, targeting technology, applied in the fields of molecular biology and medicine, can solve the problems of large molecules, weak penetrating power, and poor stability in vitro
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
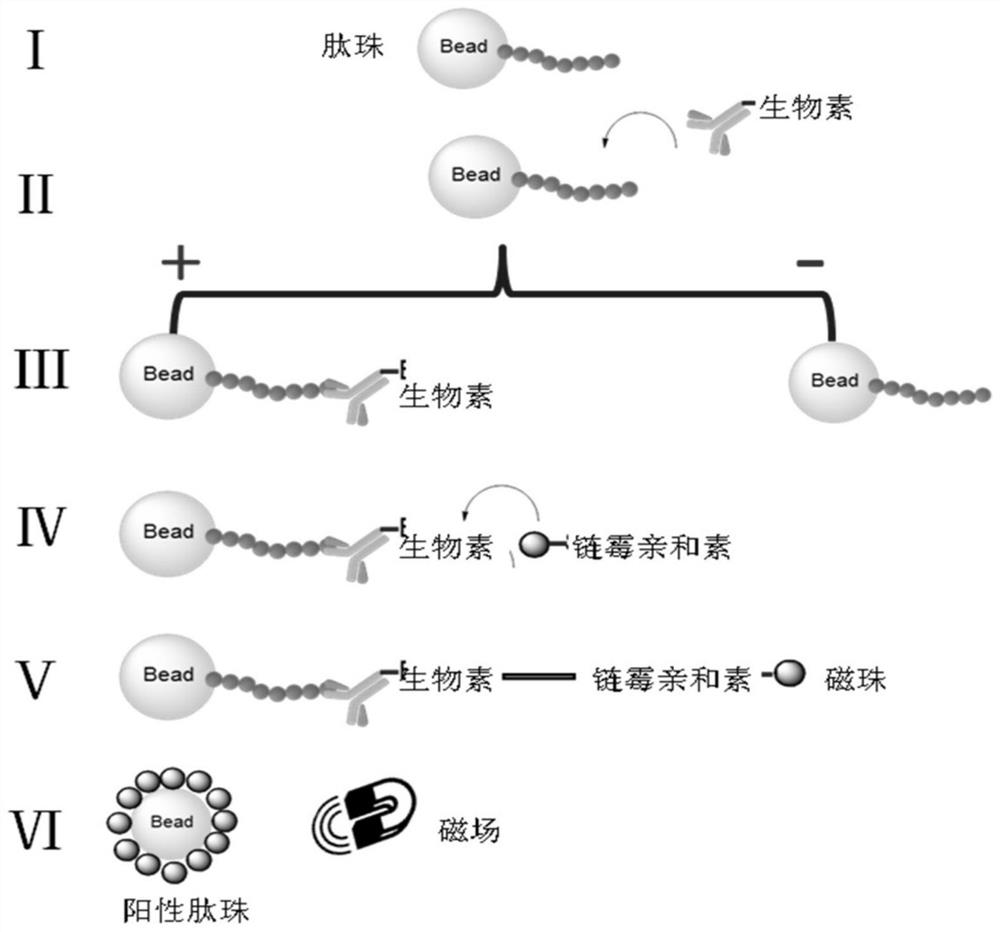
[0074] Example 1 Construction and screening of PD-L1 targeting polypeptide library
[0075] (1) Experimental instruments and materials
[0076] N-methylmorpholine (NMM), piperidine, trifluoroacetic acid (TFA), dichloromethane (DCM), ninhydrin, vitamin C, phenol, tetramethyluronium hexafluorophosphate (HBTU), hexahydro Pyridine, triisopropylsilane (TIS), ethanedithiol (EDT), N,N dimethylformamide (DMF), anhydrous ether, resin, methanol, various Fmoc protected amino acids, streptavidin Magnetic beads (MB-Streptavidin), biotin labeling kit, peptide synthesis tube, shaker, vacuum water pump, rotary evaporator, laser confocal microscope (ZEISS LSM 710), the above reagents and materials were obtained from commercial sources.
[0077] (2) Solvent preparation
[0078] The preparation of the deprotection solvent is hexahydropyridine: N, N dimethylformamide = 1:4;
[0079] The preparation of the reaction solution is N-methylmorpholine:N,N dimethylformamide=1:24;
[0080] The prepara...
Embodiment 2
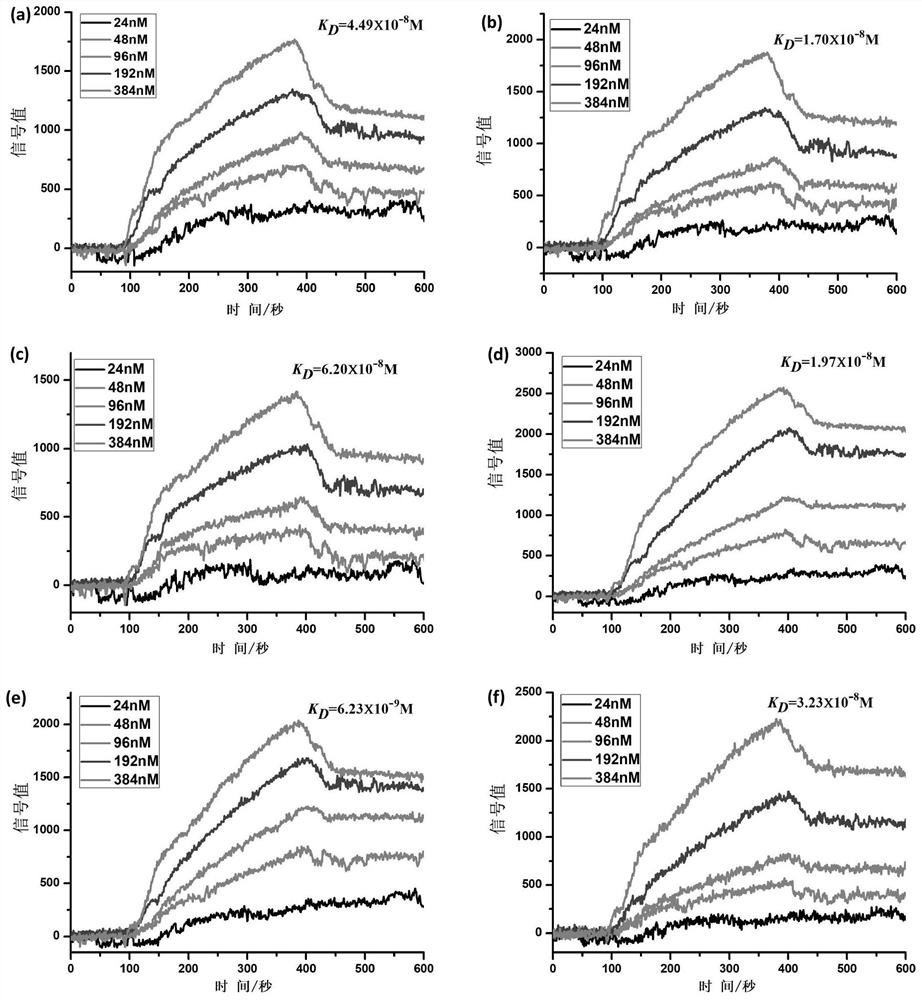
[0106] Example 2 Detection of Affinity Between Polypeptides P1, P2, P3, P4, P5, P6 and PD-L1 Protein
[0107] The affinity between polypeptides P1, P2, P3, P4, P5 and P6 and PD-L1 protein was detected by surface plasmon resonance (SPRi), the specific method is as follows:
[0108] Spot 1mg / mL P1, P2, P3, P4, P5, P6 peptides and 1×PBS on the chip, incubate overnight at 4°C under humid conditions, then wash with 10×PBS for 10 minutes, and then wash with 1×PBS for 10 minutes , and finally washed twice with deionized water, 10min each time, immersed in 1×PBS containing 5% skimmed milk, incubated overnight at 4°C, then washed with 10×PBS for 10min, 1×PBS for 10min, and finally used Washed twice with deionized water, 10min each time, blown dry with nitrogen gas, and installed the chip on the machine (Plexera HT surface plasmon resonance imaging system).
[0109] The mobile phase was sequentially passed through 1×PBS, 2×PBS, 0.625 μg / mL, 1.25 μg / mL, 2.5 μg / mL, 5 μg / mL and 10 μg / mL...
Embodiment 3
[0111] Example 3 Preparation of Imaging Preparations of Polypeptides P1, P2, P3, P4, P5, P6
[0112] This embodiment provides the preparation of imaging preparations for polypeptides P1, P2, P3, P4, P5, and P6, and the specific methods are as follows:
[0113] Weigh 300 mg of wang-Glu resin, cycle according to the solid-phase peptide synthesis procedure, first deprotect, and then add a certain amount of Glu, Asp, Glu, Thr, Trp, Lys, Asn and an equivalent amount of HTBU in sequence to react. After the coupling reaction, deprotect and wash. Add ε-aminocaproic acid to react, after coupling, deprotect and wash. Add FITC to carry out the coupling reaction in the dark, after the reaction, deprotect and wash. Finally, the lysate was added to the above resin to remove the side chain protecting group, and then vacuum-dried to obtain crude polypeptide fluorescent conjugates, and then purified by HPLC to obtain polypeptides P1, P2, P3, P4, P5, and P6 with a purity of 95%. An imaging p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com