Mitochondria targeted external medicinal preparation
A technology for topical drugs and mitochondria, which is applied in the direction of drug combination, drug delivery, and medical preparations of non-active ingredients. Permeability, promote release, help transdermal absorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
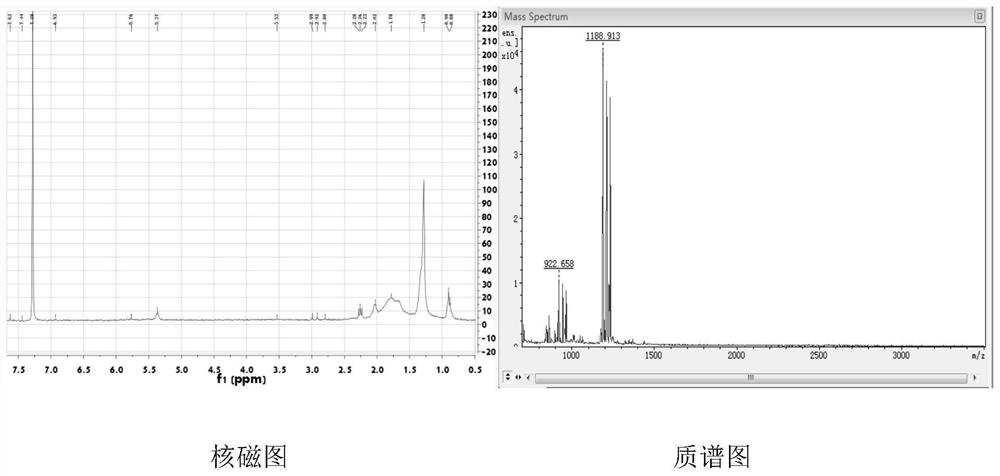
[0059] Example 1 Preparation of Peptide Dendrimers
[0060] 1. In the present invention, the mitochondrial targeting phospholipid is a drug carrier peptide dendrimer (G2), and its structural formula is:
[0061]
[0062] Its preparation method is as follows:
[0063] 1) Amidation reaction of lysine and BOC-protected arginine
[0064] According to lysine, BOC protection of arginine, condensing agent [equimolar 1-hydroxybenzotriazole (HOBT) and benzotriazole-N,N,N',N'-tetramethyluronium hexafluoro Phosphate (HBTU)], the molar ratio of the organic base is 1:3:3:1, and the metering of lysine, BOC protected arginine, HOBT, HBTU, DIPEA and DMF.
[0065] Add the above-mentioned raw materials into a branch bottle under ice bath and nitrogen protection, then add an organic base and anhydrous dimethylformamide to dissolve the solid drug, stir and react for 24-48 hours, then extract the reaction solution with ethyl acetate, and with saturated NaHCO 3 , dilute hydrochloric acid, sa...
Embodiment 2
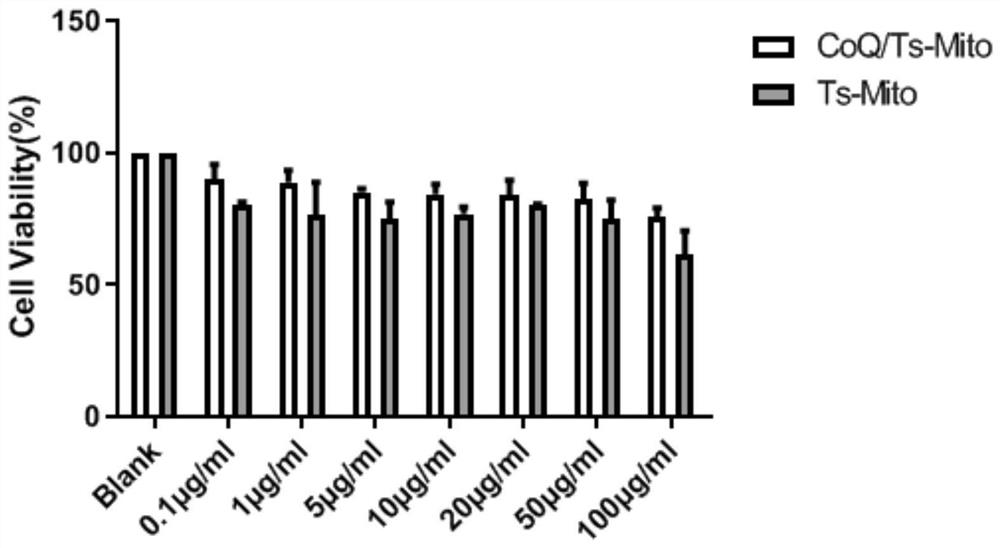
[0091] Dissolve 2 mg of the drug carrier peptide dendrimer (G2) prepared in Example 1, 6 mg of soybean lecithin, 0.5 mg of cholesterol, 1 mg of Tween 80, 1 mg of sodium deoxycholate and 1 mg of coenzyme Q10 in 25 mL of chloroform-methanol mixed solvent medium (the volume ratio of chloroform to methanol is 2:1), and then the solvent was removed by rotary evaporation under reduced pressure to obtain a dry lipid film, which was hydrated with 2 mL of deionized water, ultrasonicated in a water bath at 50°C for 15 min, followed by ultrasonic probe for 5 min, Obtain liposome solution. Then centrifuge at a speed of 3500r / min for 3min to remove free coenzyme Q10, and the obtained supernatant is the mitochondria-targeted liposome loaded with coenzyme Q10.
[0092] Dissolve 6 mg of soybean lecithin, 0.5 mg of cholesterol, 1 mg of Tween 80, 1 mg of sodium deoxycholate and 1 mg of coenzyme Q10 in 25 mL of chloroform-methanol mixed solvent (the volume ratio of chloroform and methanol is 2:1...
Embodiment 3
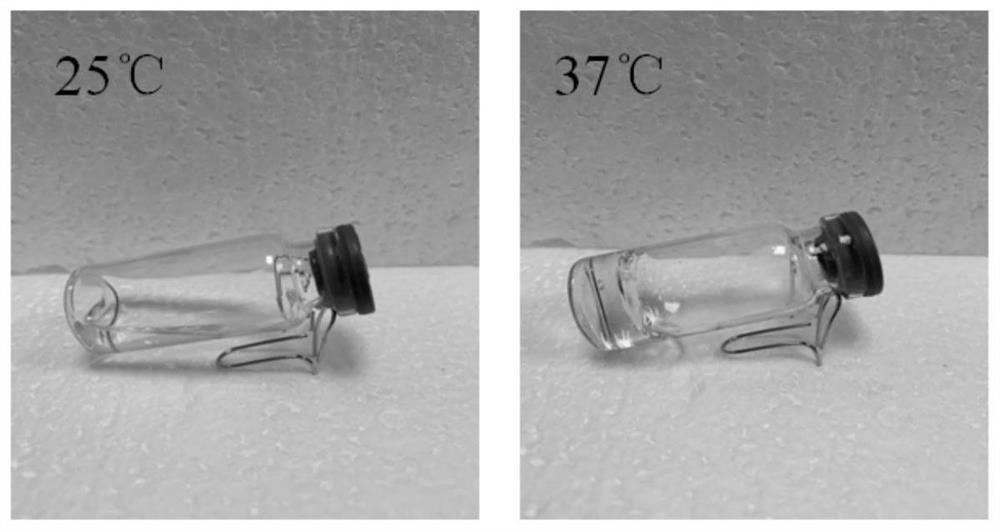
[0098] The poloxamer solution, chitosan solution and liposome solution are miscible, and the ratio of 30% poloxamer 188 and 30% poloxamer 407 to 1% low-density chitosan and liposome is 4 : 0.5: 0.5 (v: v: v), the viscous transparent fluid prepared according to this ratio forms a gel at 37°C, but cannot form a gel at room temperature and low temperature, and has heat sensitivity. The gel phase transition diagram is shown in figure 2 .
[0099] The mitochondrial targeting liposomes loaded with coenzyme Q10, the liposomes loaded with coenzyme Q10 plus surfactant, the ordinary liposomes loaded with coenzyme Q10, the mitochondrial targeted liposomes loaded with Dil, and Dil-loaded surfactant liposomes and Dil-loaded ordinary liposomes with prepared 30% poloxamer 188 and 30% poloxamer 407, 1% low-density chitosan according to poloxamer: lipid Body: Chitosan ratio 8:1:1 (v:v:v) mixed, at 37 ℃ to form the corresponding liposome gel.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com