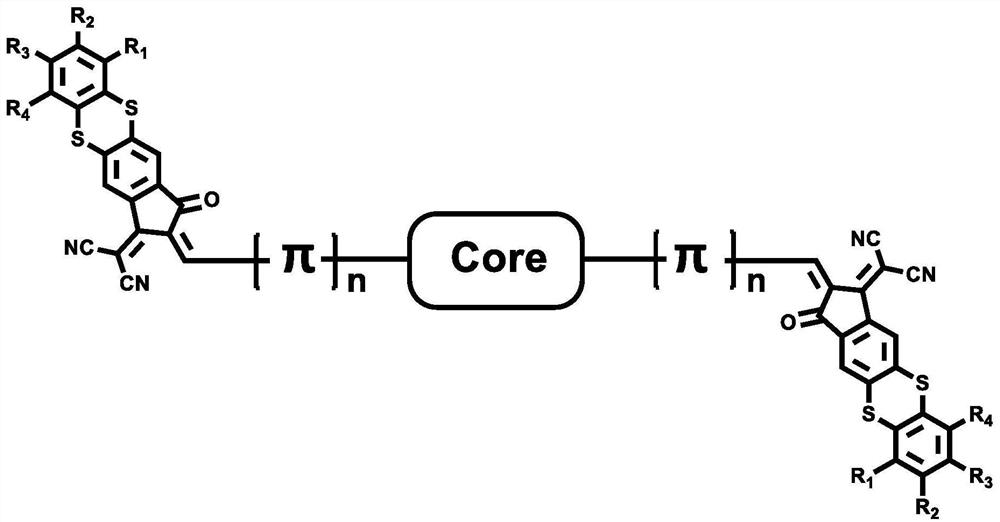
Organic conjugated small molecular material containing thianthrene end groups and preparation method thereof
A small molecule, conjugated technology, used in organic chemistry, semiconductor/solid-state device manufacturing, photovoltaic power generation, etc., can solve the problems of narrow absorption wavelength, difficult energy level regulation, high cost, and achieve high photoelectric conversion efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
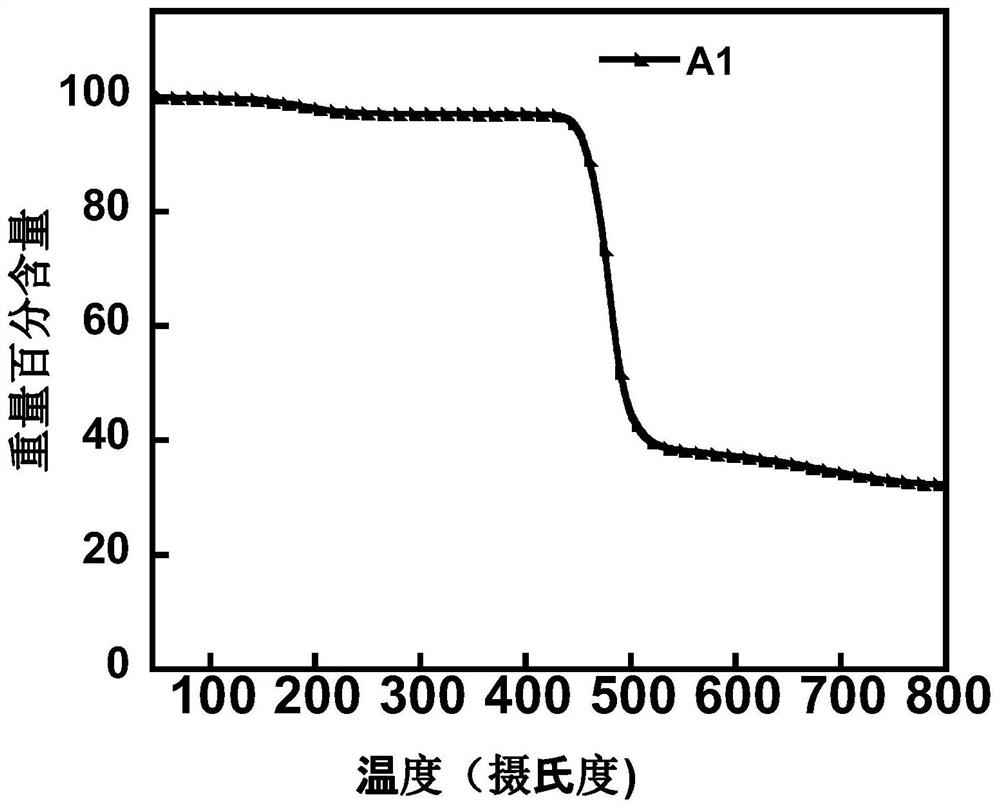
Image
Examples
Embodiment 1
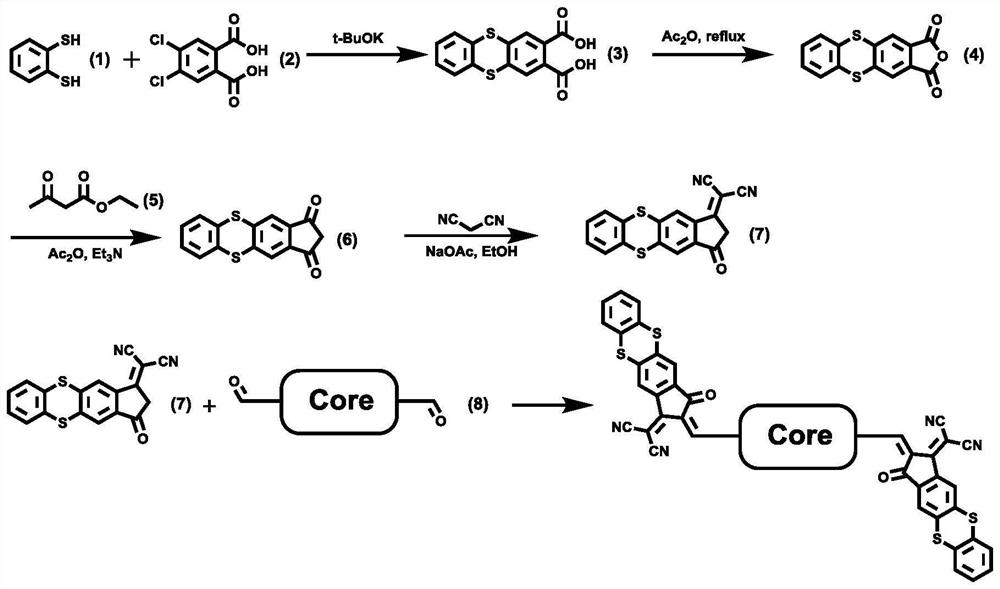
[0040] Example 1: Synthesis of compound c.
[0041] Add potassium tert-butoxide to the N,N-dimethylformamide solution of compound a and compound b, and react with stirring and heating for 12 hours. Afterwards, the reaction solution was cooled to room temperature, poured into ice water containing HCl, and a precipitate was formed. The precipitated solid compound was filtered, washed with water, dried, and then separated and purified by column chromatography to obtain the target compound c. The reaction formula is:
[0042]
Embodiment 2
[0043] Example 2: Synthesis of compound d.
[0044] The compound c and acetic anhydride were sequentially added into the reaction flask, and heated to reflux for 12 hours. Afterwards, the reaction solution was cooled to room temperature, poured into ice water, and a precipitate was formed. The precipitated solid compound was filtered, washed with water, dried, and then separated and purified by column chromatography to obtain the target compound d. The reaction formula is:
[0045]
Embodiment 3
[0046] Embodiment 3: Synthesis of compound f.
[0047] Prepare a 75mL dry pressure-proof bottle, blow it with argon, add compound d, acetic anhydride (25mL) and triethylamine (15mL) into the pressure-proof bottle in turn, and add ethyl acetoacetate quickly under stirring Into the reaction liquid, after 20 minutes of ventilation, the reaction was heated to 100°C, and the reaction was carried out for 12 hours. Afterwards, the reaction solution was cooled to room temperature, poured into ice water containing HCl, a precipitate was formed, then HCl (30 mL) was added to the mixed solution, and the mixed solution was heated to reflux, reacted for 3 hours, and then the reaction was returned to room temperature , the precipitated solid compound was filtered, washed with water, dried, and then separated and purified by column chromatography, and the eluent was dichloromethane to obtain the target compound f. The reaction formula is:
[0048]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com