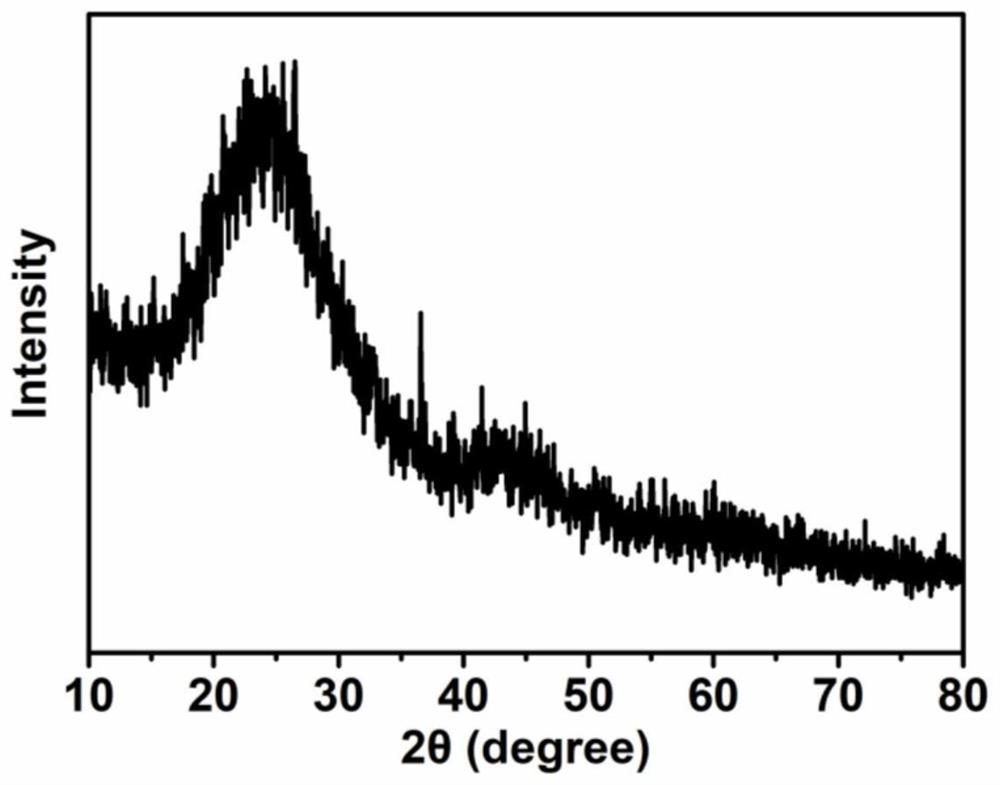
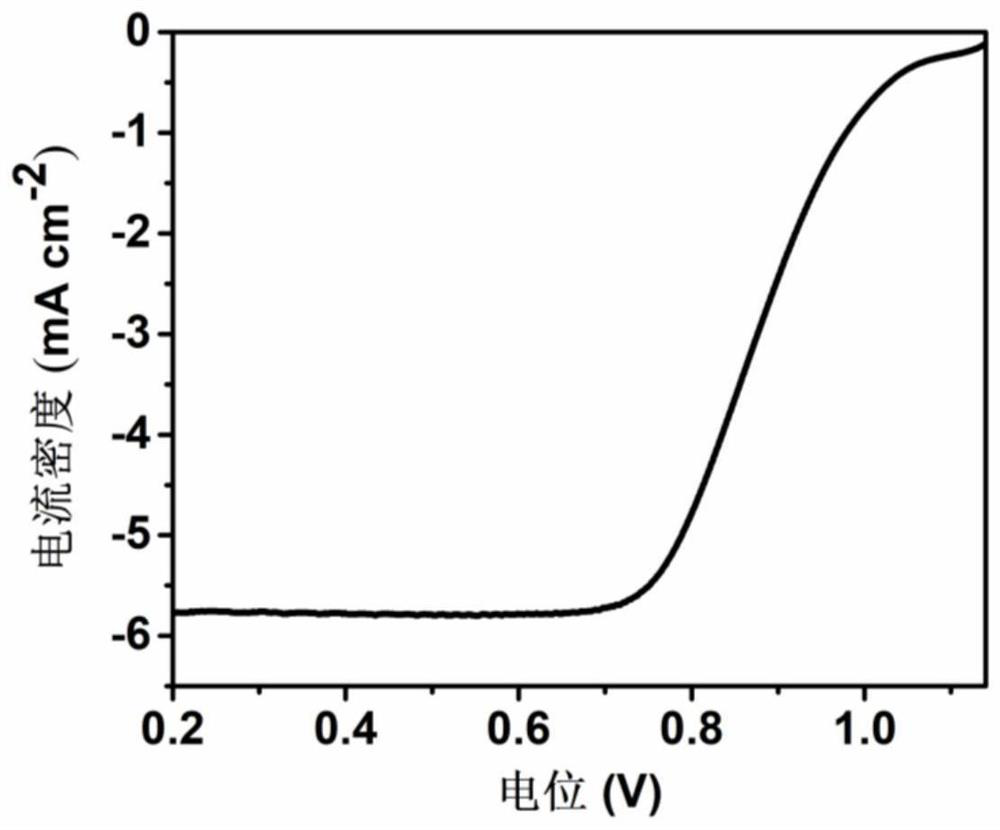
Preparation method of pyrrole-derived monatomic iron-based nitrogen-carbon material for oxygen reduction
An iron-based nitrogen and carbon material technology, applied in chemical instruments and methods, physical/chemical process catalysts, chemical/physical processes, etc., can solve the problems of easy agglomeration of metal atoms, complicated preparation process, low current density, etc. The effect of good performance, simple operation and high limiting current density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] A general preparation method of a single-atom iron-based nitrogen-carbon material and its application as a good catalyst for oxygen reduction, the preparation steps are as follows:
[0030] (1) adding an iron source compound and a zinc source compound to the hydrophilic solution to obtain a metal-organic solution of a certain concentration;
[0031] (2) add polymer monomer to the mixed system in step 1;
[0032] (3) stirring and reacting the mixed system in step 2 for a certain period of time to obtain a reaction product, washing and drying the product to obtain a metal-containing polymer;
[0033] (4) The product obtained in step 3 is calcined at a certain temperature for a certain period of time, and then naturally cooled to room temperature to prepare a monoatomic iron-based nitrogen-carbon material.
Embodiment 1
[0035] (1) Take a clean 200mL beaker and add 100mL of anhydrous methanol to the beaker.
[0036] (2) 500 mg of ferric nitrate nonahydrate and 15 g of zinc nitrate hexahydrate were simultaneously added to the methanol solution in step (1), and an orange clear solution was obtained under stirring.
[0037] (3) Add 1000 μL of pyrrole to the clear solution obtained in step 2, and react under stirring for 24 hours to obtain a dark blue turbid solution.
[0038] (4) The turbid solution in step (3) was centrifuged and washed several times with ethanol and deionized water, and the product obtained by suction filtration was dried at 80° C. for 12 hours.
[0039] (5) Calcining the dried product obtained in step (4) at 900° C. for 3 h under an argon atmosphere.
Embodiment 2
[0041] (1) Take a clean 200mL beaker and add 100mL of anhydrous methanol to the beaker.
[0042] (2) Add 500 mg of ferric nitrate nonahydrate into the methanol solution in step (1), and stir to obtain an orange clear solution.
[0043] (3) Add 1000 μL of pyrrole to the clear solution obtained in step 2, and react under stirring for 24 hours to obtain a dark blue turbid solution.
[0044] (4) The turbid solution in step (3) was centrifuged and washed several times with ethanol and deionized water, and the product obtained by suction filtration was dried at 80° C. for 12 hours.
[0045] (5) Calcining the dried product obtained in step (4) at 900° C. for 3 h under an argon atmosphere.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com