Recombinant escherichia coli capable of producing O-acetyl-L-homoserine at high yield and application of recombinant escherichia coli
A technology of recombinant Escherichia coli, homoserine, applied in the field of metabolic engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Construction of plasmid pTrc99A-metX expressing L-homoserine acetyltransferase
[0035] Using the genome of wild-type Corynebacterium glutamicum ATCC13032 as a template, together with primers MetX-F and primers MetX-R, PCR amplification was carried out. PCR reaction conditions: pre-denaturation at 95°C for 5min, 95°C for 30s, 60°C for 30s, 72°C for 1min, a total of 30 cycles, and finally extension at 72°C for 10min. The PCR product was detected by 1.0% agarose gel electrophoresis, and the fragment was recovered and purified by cutting the gel.
[0036] The empty vector pTrc99A was used as a template, together with primers pTrc99A-F and primers pTrc99A-R for PCR amplification. PCR reaction conditions: pre-denaturation at 95°C for 5min, 95°C for 30s, 60°C for 30s, 72°C for 4min, a total of 30 cycles, and finally extension at 72°C for 10min. The PCR products were detected by 1.0% agarose gel electrophoresis, and the gel-cut gel was used to recover and purify t...
Embodiment 2
[0040] Embodiment 2: Construction of bacterial strain OAH-1
[0041]Using CRISPR-Cas9 gene editing technology, the metJ, metI, metB, thrB, metA, lysA and iclR genes in E. coli W3110 (purchased from The Coli Genetic Stock Center) were knocked out to obtain large intestine E. coli W3110ΔmetJΔmetIΔmetBΔthrBΔmetAΔlysAΔiclR (Peng Liu et al. 2020. Multiplex design of metabolic network for production of L-homoserine in Escherichia coli. Applied and Environmental Microbiology). Escherichia coli W3110ΔmetJΔmetIΔmetBΔthrBΔmetAΔlysAΔiclR was used as the starting strain, and the CRISPR-Cas9 gene editing technology was used (Yu Jiang et al.2015Multigene Editing in the Escherichia coli Genome via the CRISPR-Cas9 System.Applied Environmental Microbiology.81,2506-r4tA). The in situ promoters of the thrA and eamA genes were replaced by the trc promoter to obtain the recombinant strain OAH-1:
[0042] Construction of pTarget plasmid: Using the pTarget F plasmid (Addgene Plasmid#62226) as a tem...
Embodiment 3
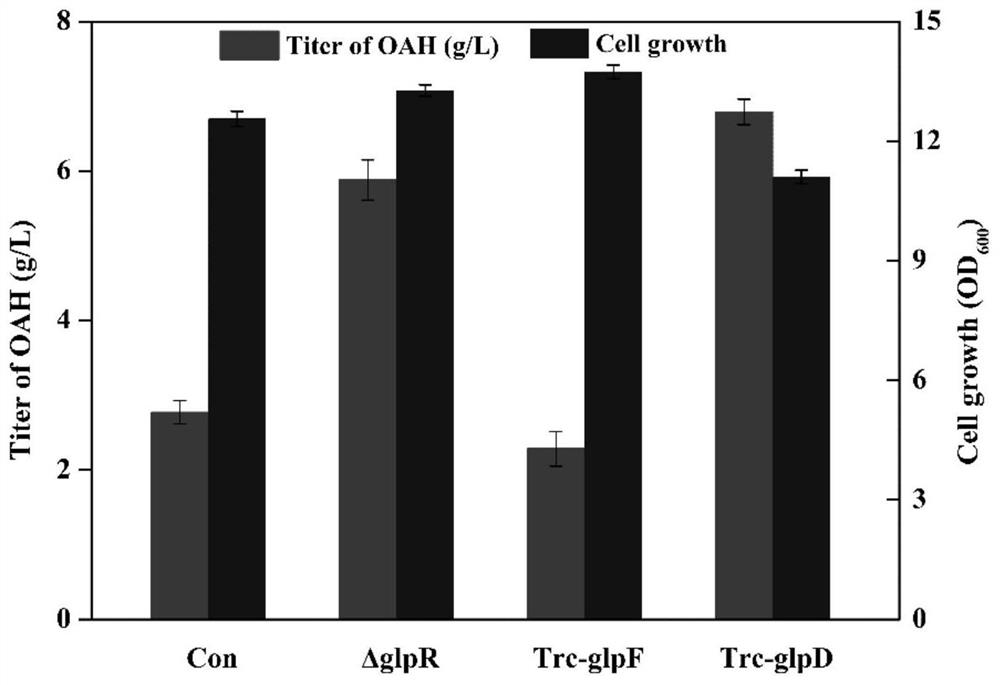
[0046] Example 3: Construction and Shake Flask Fermentation of Strains OAH-2 / pTrc99A-metX, OAH-3 / pTrc99A-metX, OAH-4 / pTrc99A-metX Enhancing the Flux of Glycerol Metabolic Pathways to Produce O-Acetyl-L-Homoserine
[0047] Starting from the strain OAH-1 (W3110, ΔmetJΔmetIΔmetBΔthrBΔmetAΔlysAΔlacI::Trc-rhtAΔiclR Trc-metL Trc-thrA Trc-rhtA Trc-eamA), the gene editing technology mediated by CRISPR-Cas9 (Yu Jiang et al.2015Multigene Editing in the Escherichia coliGenome via the CRISPR-Cas9 System.Applied Environmental Microbiology.81:2506-2514), respectively knock out the glpR gene, overexpress the glpF gene and overexpress the glpD gene (the method is the same as in Example 2), and obtain bacterial strain OAH-2, OAH -3 and OAH-4. Transform the constructed plasmid pTrc99A-metX into the above-mentioned genetic engineering bacteria and the control strain OAH-1 to obtain recombinant strains OAH-1 / pTrc99A-metX, OAH-2 / pTrc99A-metX, OAH-3 / pTrc99A-metX and OAH -4 / pTrc99A-metX.
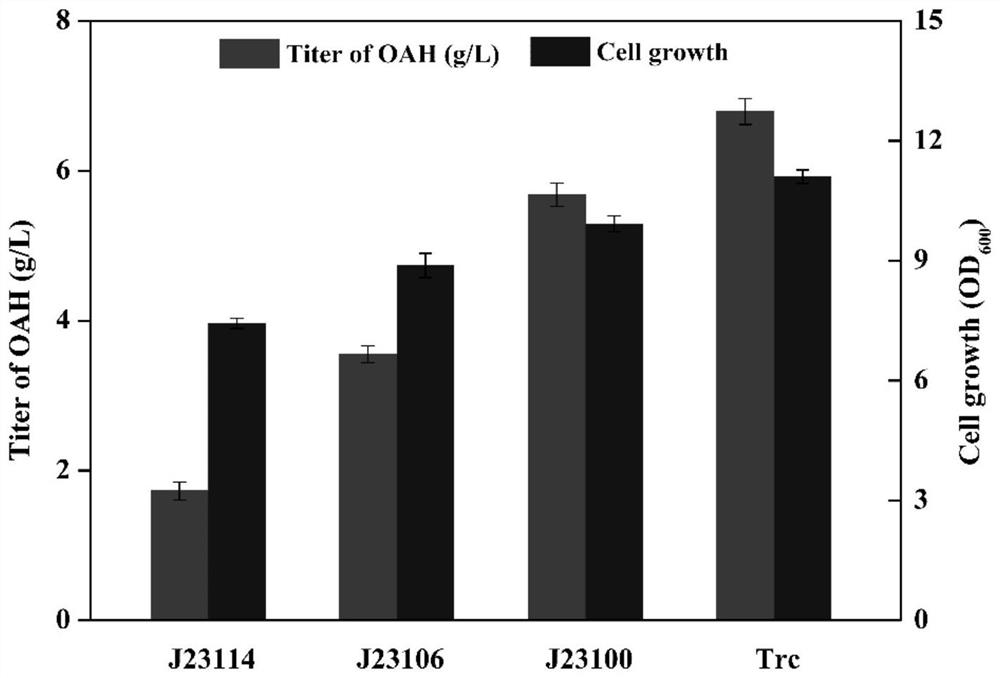
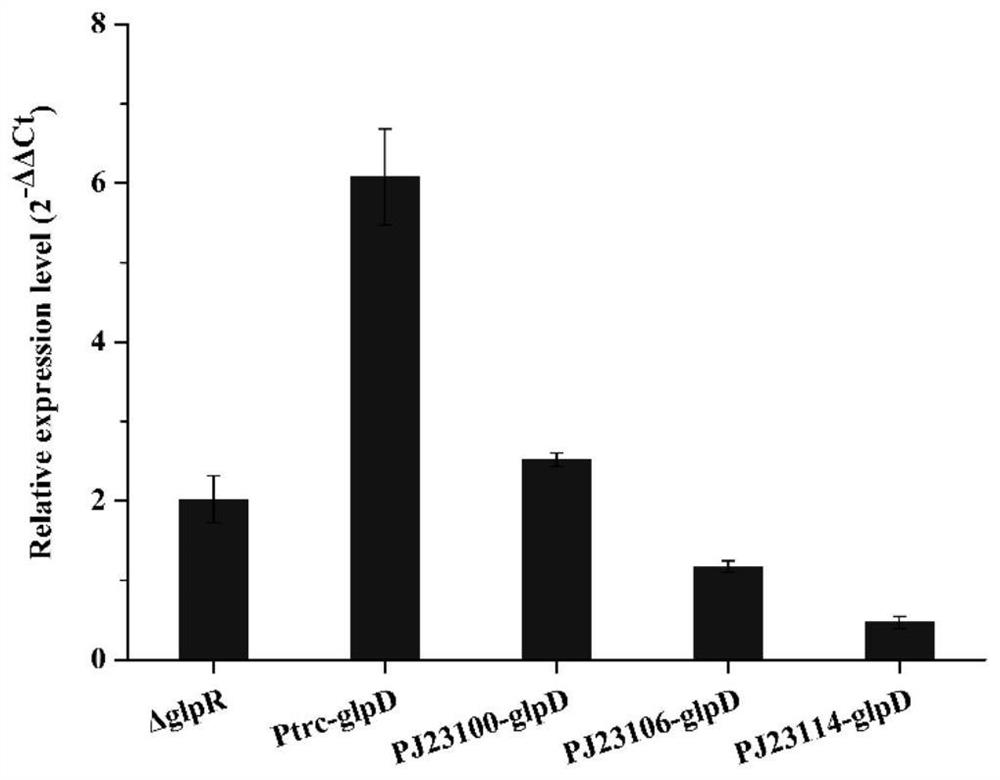
[0048] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com