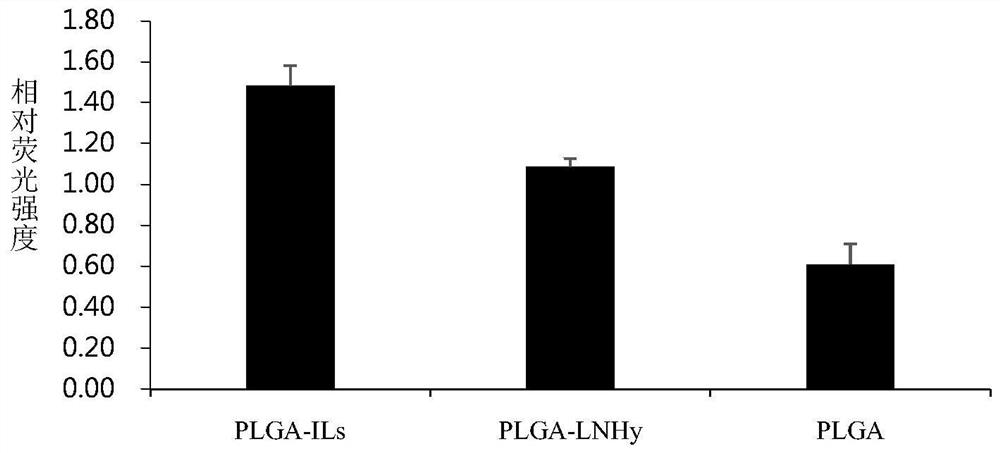
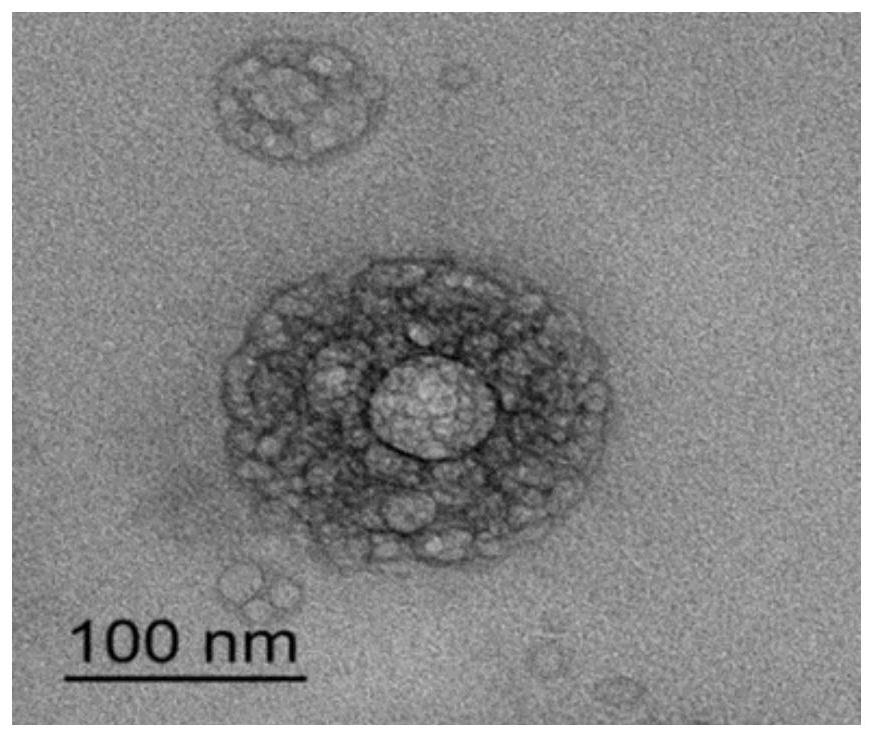
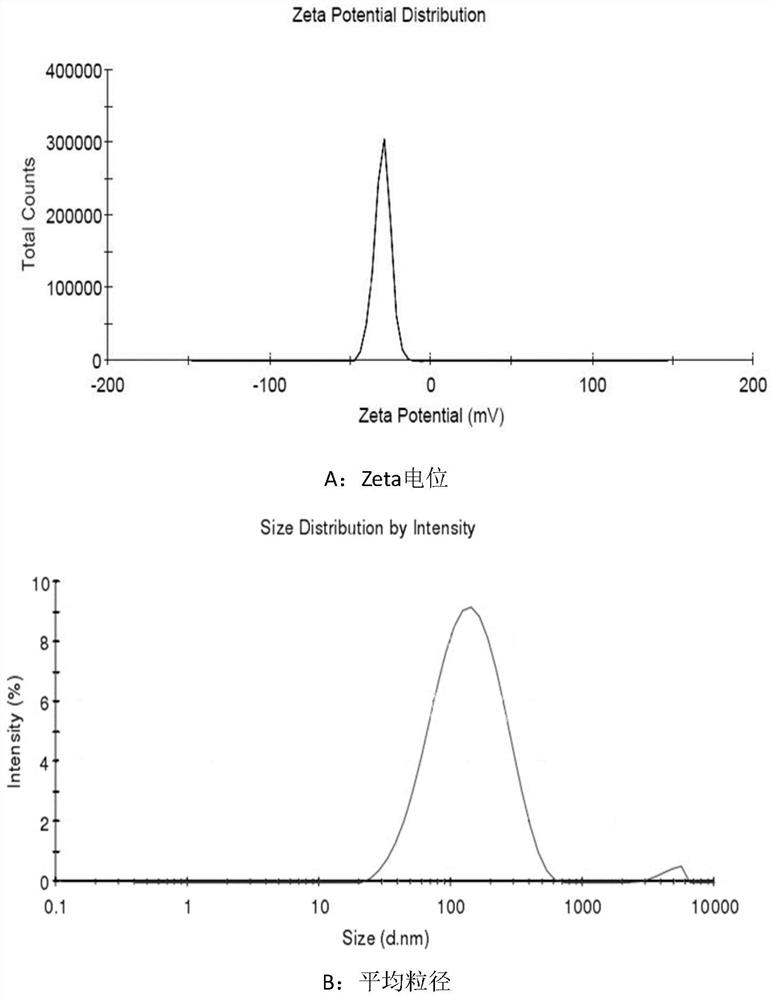
Liposome-nanoparticle hybrids for treating chronic glomerulonephritis
A nanoparticle and complex technology, applied in the field of medicine, can solve the problems of low drug loading, low encapsulation rate, unfavorable CGN and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Example 1 Integrin α8-modified liposome-PLGA nanoparticle complex PLGA-ILs / double drug loaded with double drug
[0079] (1) Preparation of nano core
[0080] Precisely measure 10mg PLGA and 3mg dexamethasone (DXMS) dissolved in 3mL acetone solution and vortex mix as the organic phase solution, and drop the organic phase solution into 15mL 1% poise The aqueous phase solution of Loxamer 188 (vortex stirring 1000r / min), stirred at room temperature for 15min, and was then rotary evaporated in a water bath at 37°C for 10min to remove the organic phase. To remove nanoparticles with larger particle size, centrifuge the solution at 8000r / min for 5min, recover the supernatant, and then centrifuge the recovered supernatant at 12000r / min for 10min, remove the free DXMS from the supernatant, and add 10mL of deionized water Resuspended to obtain the nano-core (PLGA / DXMS).
[0081] (2) Preparation of PLGA-LNHy / double drug
[0082] The membrane materials DOPE, DOTAP, cholesterol (C...
Embodiment 2
[0085] Example 2 Liposome-gold nanoparticle complex (Au-ILs / siRNA / DXMS) modified by integrinα8 loaded with siRNA / DXMS
[0086] (1) Preparation of nano core
[0087] Synthesis of siRNA: Design, synthesize and screen the effective fragment TGF-β1siRNA of the target gene. The synthesized target gene fragment must have a sulfhydryl group to make it easy to bind with AuNPs.
[0088] Precisely measure an appropriate amount of 0.11mg / mL of HAuCl 4 4H 2 O solution, stirred and heated to boiling, slowly added a certain volume of 34mM sodium citrate solution, continued to stir and heat for 10min, and then cooled and stirred for 15min to obtain a red AuNPs solution, which was sealed at 4°C and stored away from light.
[0089] Preparation of AuNPs-siRNA complex: Dilute 10pmol siRNA and AuNPs according to (1:0 or 1:1 or 1:2 or 1:5 or 1:10 or 1:20) different mass ratios, mix them, and store them at 37°C Incubate for 30 min to prepare AuNPs-siRNA complex, transfer the reaction product int...
Embodiment 3
[0094] Example 3 Integrin α8-modified liposome-MS nanoparticle complex (MS-ILs / two different model drugs) loaded with double drugs
[0095] (1) Preparation of nano core
[0096] Weigh a certain amount of cetyltrimethylammonium bromide (CTAB) and dissolve it in deionized water, add 0.3g sodium acetate, appropriate amount of ethanol and 1mol L -1 sodium hydroxide, stirred and heated up to a certain temperature until the solution was clear and transparent, added an appropriate amount of tetraethyl orthosilicate (TEOS) dropwise under stirring, stirred at a constant temperature and high speed for 2 hours, and then repeatedly centrifuged and washed with an appropriate amount of water and ethanol solution, Dry at room temperature. Weigh an appropriate amount of dried powder and suspend it in a concentrated hydrochloric acid-ethanol mixture (5:90) at 85°C for 24 hours at 85°C for 24 hours, continue to use appropriate amount of water and ethanol solution to repeatedly centrifuge and w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com