Pillararene and pillararene-like compounds with aggregation-induced luminescent effect, preparation method and application thereof
A technology of aggregation-induced luminescence and column aromatics, which is applied to non-active ingredients in medical preparations, hydrocarbons, hydrocarbons, etc. It can solve problems such as not many reports, and achieve improved quantum yield and excellent aggregation induction. effect of glow effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
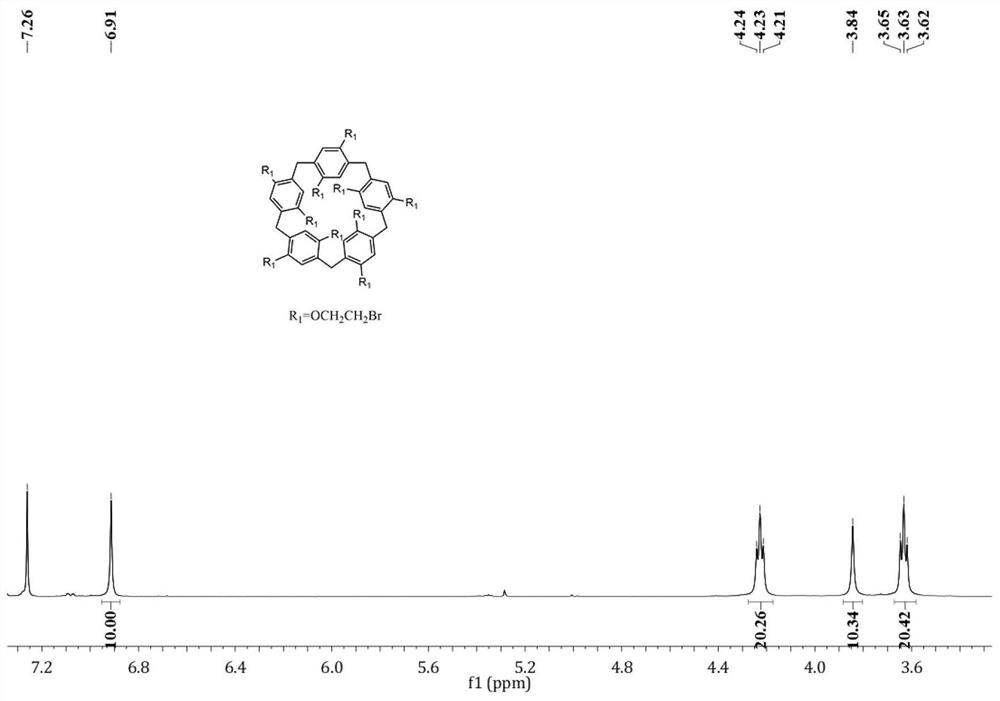
[0078] Synthesis of monocyclic quaternary ammonium salt cationic water-soluble pillararene with aggregation-induced luminescent effect, the synthetic route is as follows:
[0079]
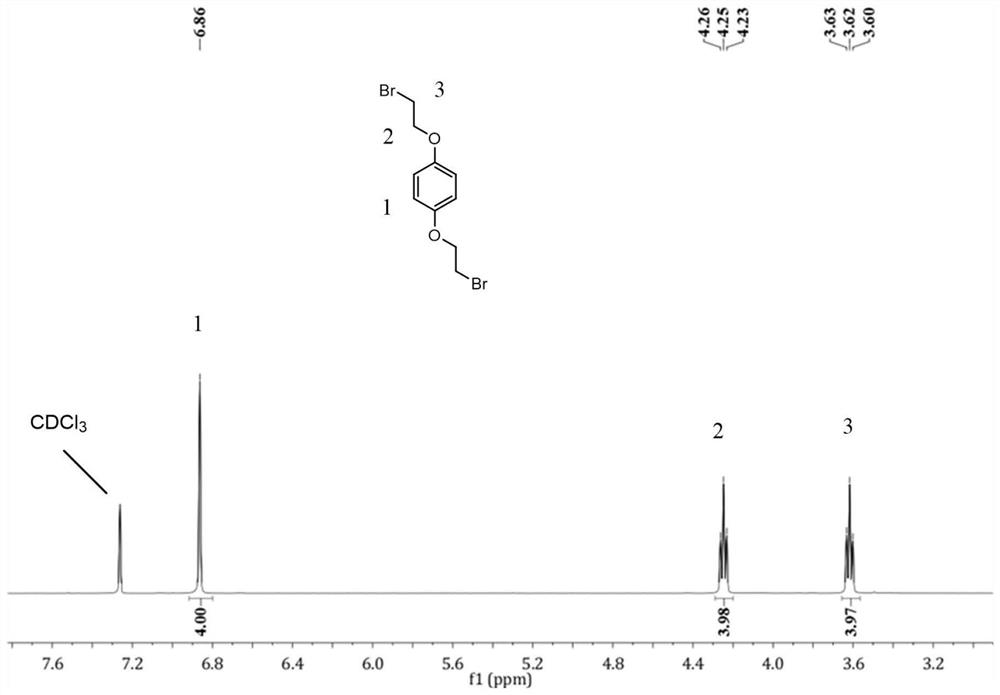
[0080] (1) Synthesis of 1,4-bis(2-bromoethoxy)benzene: Add hydroquinone dihydroxy ether and triphenylphosphine to acetonitrile (or acetone) at a molar ratio of 1:3, at 0°C , add carbon tetrabromide in batches (the molar ratio of hydroquinone dihydroxy ether to carbon tetrabromide is 1:3), continue to stir at room temperature for 24h, add deionized water to quench, filter, wash, and column chromatography Separated to obtain a white powdery solid which is 1,4-bis(2-bromoethoxy)benzene. The reaction can also be quenched by adding deionized water, suction filtered and washed with a mixed solution of methanol and water to obtain 1,4-bis(2-bromoethoxy)benzene. Hydrogen spectra such as figure 1 as shown, 1 H NMR (400MHz, CDCl 3 ) δ (ppm): 6.86 (s, 4H), 4.25 (t, J = 6.2Hz, 4H), 3.62 (t, J = 6.2Hz, 4...
Embodiment 2
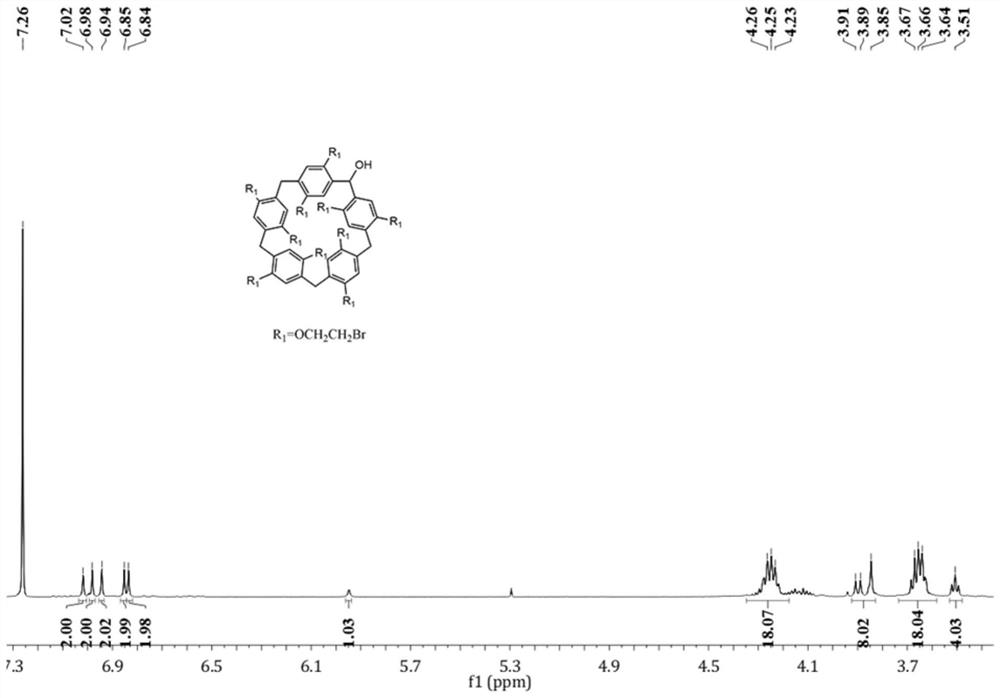
[0088] Synthesis of monocyclic pillar aromatics with aggregation-induced luminescent effect, the synthetic route is as follows:
[0089]
[0090] (1) Synthesis of compound A: Add 1,4-dimethoxybenzene and paraformaldehyde to dichloromethane (or trichloromethane) at a molar ratio of 1:3, and add catalyst trifluorination at 0°C Boron ether, the molar ratio of boron trifluoride ether to 1,4-dimethoxybenzene is 1:1, react at room temperature for 4 hours, add deionized water to quench, extract by separation, dry, and purify by column chromatography to obtain White powdery solid dimethoxypillar[5]arene (compound A).
[0091] (2) Synthesis of compound B: in N 2 Under protection, compound A and boron tribromide were added to chloroform at a molar ratio of 1:3, and reacted at room temperature for 3 days. It was quenched with water, washed, and dried in vacuo to give dihydroxycylind[5]arene (compound B) as a white solid.
[0092] (3) Synthesis of compound C: in N 2 Under protectio...
Embodiment 3
[0098] Synthesis of monocyclic pillar aromatics with aggregation-induced luminescent effect, the synthetic route is as follows:
[0099]
[0100] Synthesis of compound 5a: Add compound 3a, zinc powder and compound 3b to tetrahydrofuran at a molar ratio of 1:40:10, add titanium tetrachloride at 0°C, and the molar ratio of compound 3a to titanium tetrachloride is 1:20 Refluxed overnight, quenched, suction filtered, separated and extracted, dried, and purified by column chromatography to obtain a white solid (compound 5a).
[0101] The synthesis method of compound 3a is the same as that of Example 1, and the synthesis method of compound 3b is the same as that of Example 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com