H2S near-infrared fluorescent molecular probe as well as preparation method and application thereof
A fluorescent molecular probe and near-infrared technology, applied in fluorescence/phosphorescence, chemical instruments and methods, and material analysis through optical means, can solve problems such as fluorescence interference, achieve short response time, low cytotoxicity, and reduce interference Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
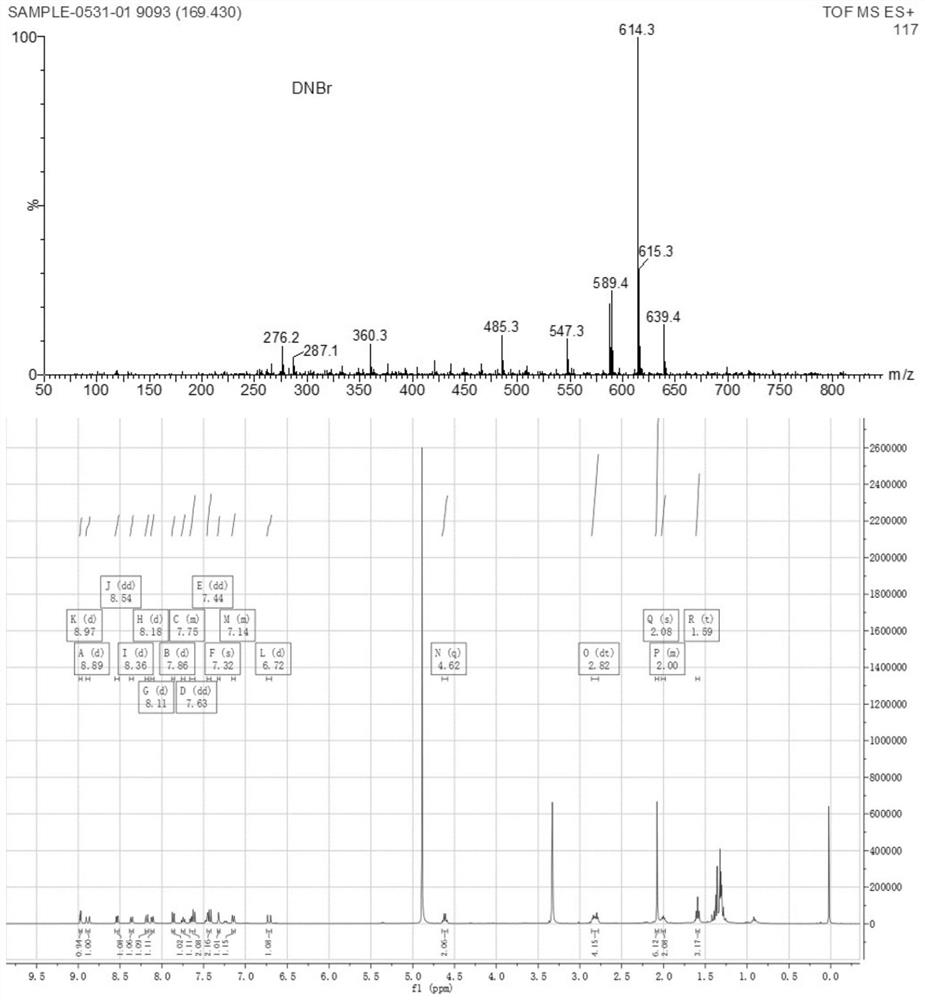
[0040] Preparation of near-infrared fluorescent probes for detecting hydrogen sulfide:
[0041] Compound 1 (1mmol) was added to a round-bottomed flask, N,N-dimethylformamide (12mL) was slowly added under nitrogen protection, stirred to dissolve completely, triethylamine (3mmol) was added under nitrogen protection, Continue to stir for 10 min, slowly add 3,5-dinitrobromobenzene (3 mmol) under nitrogen protection, and stir to dissolve completely. Put the above system in an oil bath, stir and heat to 85°C, react for 8 hours, wait until the solution turns dark blue, cool the system, pour the reaction solution in the bottle into a separatory funnel, add dichloromethane and distilled water, shake fully, and let stand Separating funnel, after the solution in the separating funnel is separated into layers, remove the lower organic layer. The above operation was repeated three times, and the organic layers were combined. The resulting solution was spin-dried to obtain a dark blue sol...
Embodiment 2
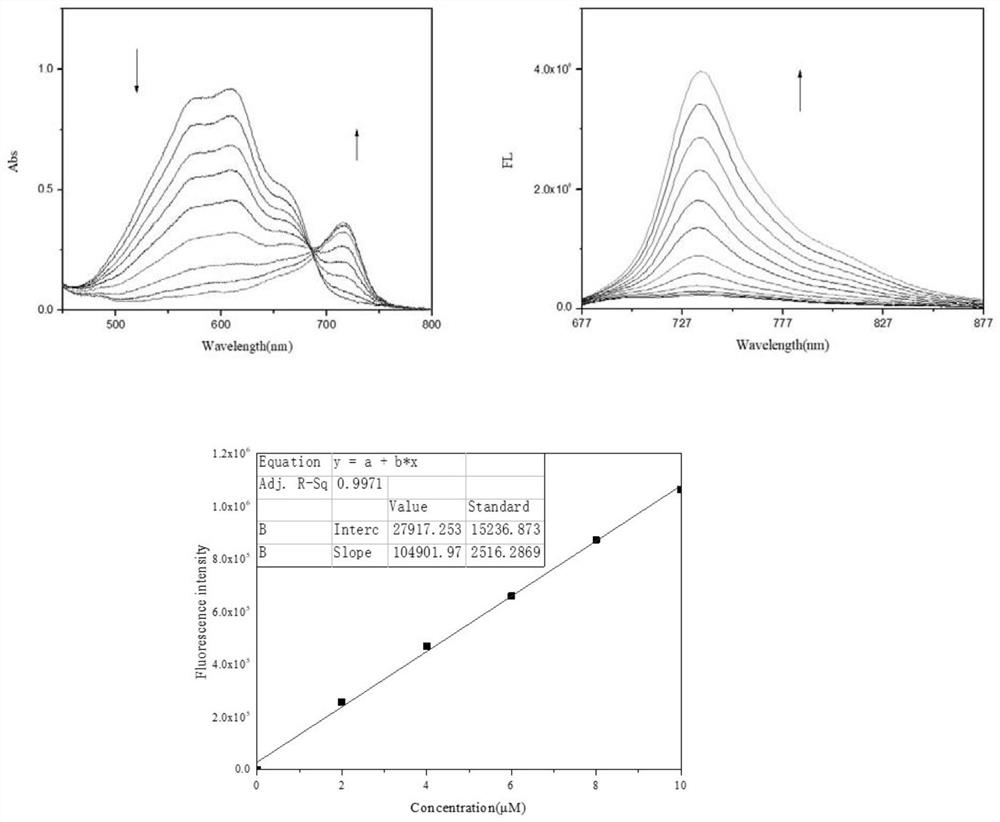
[0044] Near-infrared fluorescent probes and H 2 S in vitro response UV and fluorescence response experiments:
[0045] Weigh 6.12 mg (10 μmol) of the probe (NRh-DNBR) prepared in Example 1 and dissolve it in 1 mL DMSO to prepare a 10 mM probe stock solution. Weigh 5.6mg NaHS and dissolve it in 1mL water to make 100mM NaHS mother solution. 3 μL was drawn from the 10 mM probe mother solution and added to a cuvette filled with 3 mL of PBS buffer solution (pH=7.4), at which time the probe concentration was 10 μM. In the cuvette, add NaHS (final concentration 0-200μM) for ultraviolet and fluorescence response experiments (excitation light wavelength is 680nm), and the obtained data are processed by origin software. figure 2 , as shown in the figure, the UV absorption maximum of the probe before adding NaHS is at 680nm, and the fluorescence emission at 732nm is very low. After NaHS was added dropwise, the ultraviolet peak at 680nm decreased, the ultraviolet absorption peak at 71...
Embodiment 3
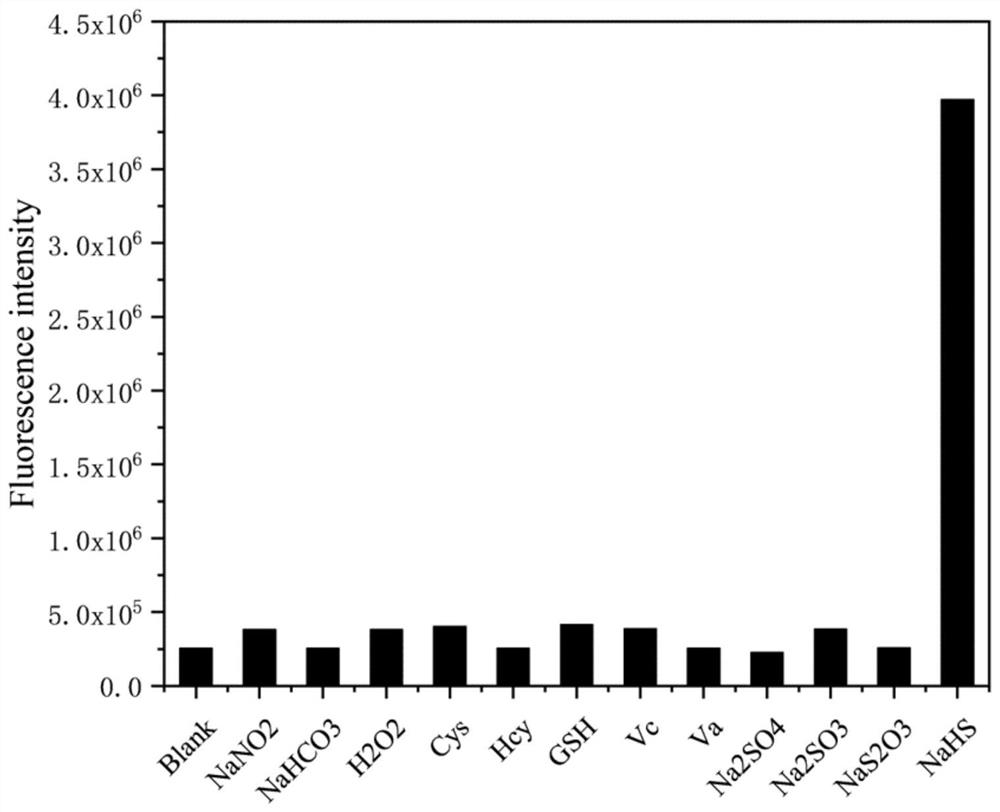
[0048] Optional experiment:
[0049] The NRh-DNBR probe (1 mM) and substrate (100 mM) prepared in Example 1 were prepared in DMSO. 30 μL was taken from each 100 mM substrate solution, respectively added to 12 centrifuge tubes containing 2940 μL of PBS buffer (pH=7.4) and mixed. Then 30 μL was taken from the 1 mM probe solution and added to each substrate solution respectively. The substrate to be tested is NaNO 2 , NaHCO 3 、H 2 o 2 , Cys, Hcy, GSH, Vc, Va, Na 2 SO 4 、Na 2 SO 3 、Na 2 S 2 o 3 , NaHS. Fluorescence emission spectra were measured with a four-color dish. The result is as image 3 As shown, only the NaHS group showed a strong fluorescent signal, that is, the fluorescent probe of the present invention was paired with H 2 S has good selectivity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com