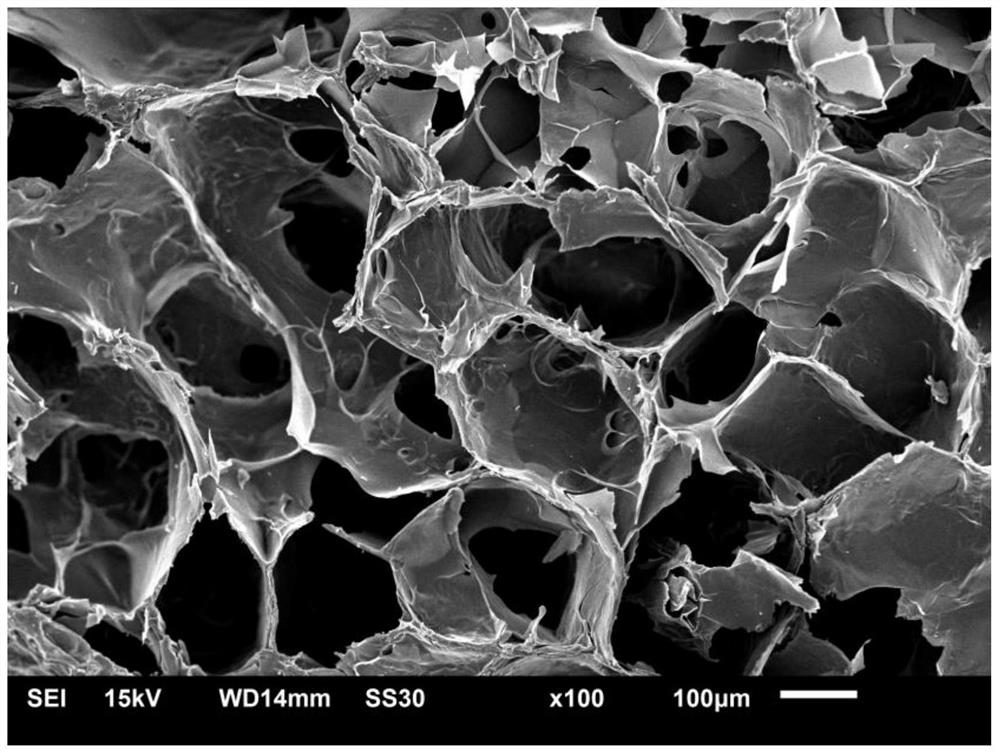
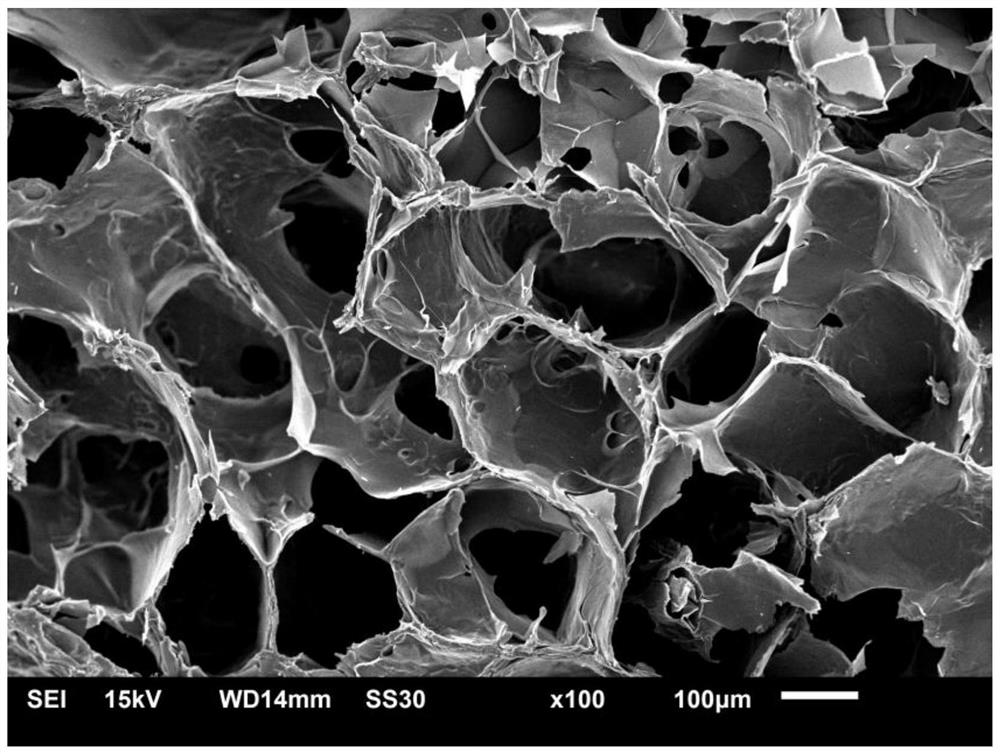
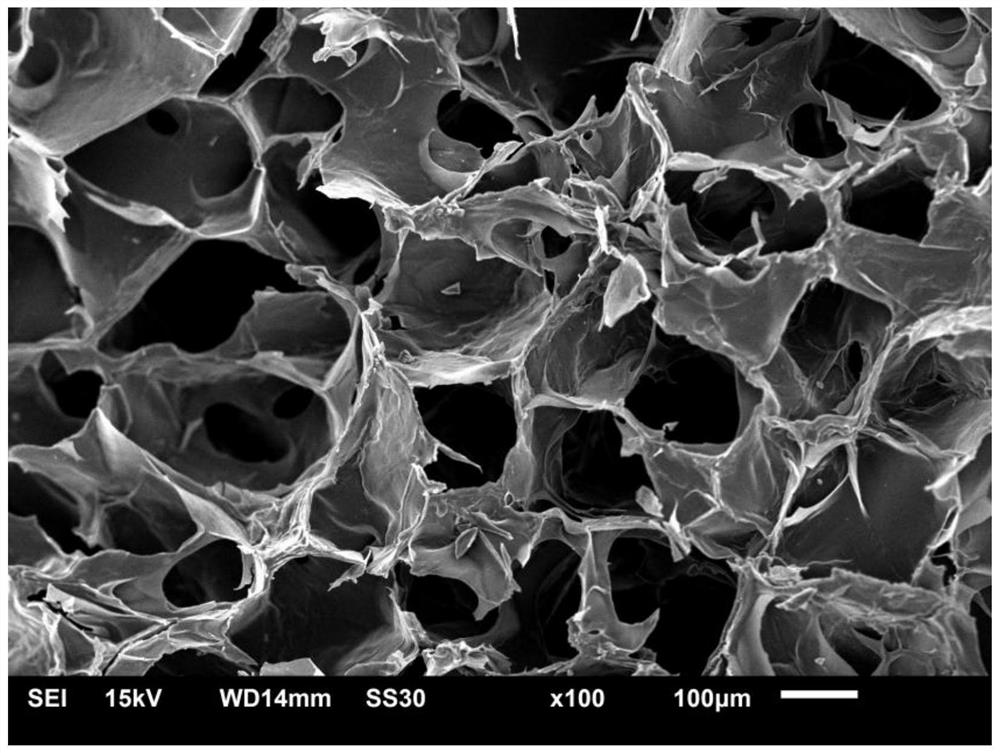
3D-printable epsilon-polylysine antibacterial hydrogel as well as preparation method and application of 3D-printable epsilon-polylysine antibacterial hydrogel
A polylysine, 3D printing technology, used in medical science, prosthesis, additive processing, etc., can solve the problems of hydrogel cytotoxicity, insufficient mechanical strength, poor biocompatibility, etc. The effect of fast glue speed and mild operating conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] (1) Add methacrylic acid in deionized water at 65°C. After it is completely dissolved, add the molar ratio of EDC, NHS and MA to the solution at 25°C to activate the carboxyl group on the methacrylic acid. For 30 minutes, add ε-polylysine (ε-PL, molecular weight 3700 Daltons) into the mixture at 40°C, the mass concentration of ε-PL is 10g / L, stir to dissolve, ε-PL and MA The molar ratio was 1:1, the pH value of the reaction system was adjusted to 4.5, and the reaction was carried out at 40°C for 15h. The obtained polymer was transferred to a dialysis bag, placed in deionized water and dialyzed for 3 days, and the purified solution obtained after dialysis was freeze-dried to obtain a methacrylic acid-modified ε-polylysine polymer (ε-PL-MA) , the grafting rate was 13.4%.
[0047] (2) carboxymethyl cellulose is dissolved in deionized water, the mass concentration of CMC is 10g / L, stirring and dissolving; Then add glycidyl methacrylate (GMA), the mol ratio of CMC and GMA i...
Embodiment 2
[0050] (1) Add methacrylic acid in deionized water at 65°C. After it is completely dissolved, add the molar ratio of EDC and NHS to MA into the solution at 25°C to activate the activation of methacrylic acid. Carboxyl for 30 minutes, add ε-polylysine (ε-PL, molecular weight 3600 Daltons) into the mixture at 45°C, the mass concentration of ε-PL is 10g / L, stir to dissolve, ε-PL and The molar ratio of MA was 1:2, the pH value of the reaction system was adjusted to 5, and the reaction was carried out at 45°C for 20h. The obtained polymer was transferred to a dialysis bag, placed in deionized water and dialyzed for 3 days, and the purified solution obtained after dialysis was freeze-dried to obtain a methacrylic acid-modified ε-polylysine polymer (ε-PL-MA) , the grafting rate was 16.3%.
[0051] (2) carboxymethyl cellulose is dissolved in deionized water, the mass concentration of CMC is 20g / L, stirring and dissolving; Then add glycidyl methacrylate (GMA), the mol ratio of CMC and...
Embodiment 3
[0054] (1) Add methacrylic acid in deionized water at 65°C. After it is completely dissolved, add it into the solution at 25°C according to the molar ratio of EDC / NHS and MA at 1:1.5 to activate the activation of methacrylic acid. Carboxyl group for 30 minutes, add ε-polylysine (ε-PL, molecular weight 4000 Daltons) into the mixture at 50°C, the mass concentration of ε-PL is 20g / L, stir to dissolve, ε-PL and The molar ratio of MA was 1:2, the pH value of the reaction system was adjusted to 4, and the reaction was carried out at 50°C for 24h. The obtained polymer was transferred to a dialysis bag, placed in deionized water and dialyzed for 5 days, and the obtained purified solution after dialysis was freeze-dried to obtain a methacrylic acid-modified ε-polylysine polymer (ε-PL-MA) , the grafting rate was 18.7%.
[0055] (2) carboxymethyl cellulose is dissolved in deionized water, the mass concentration of CMC is 10g / L, stirring and dissolving; Then add glycidyl methacrylate (GM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com