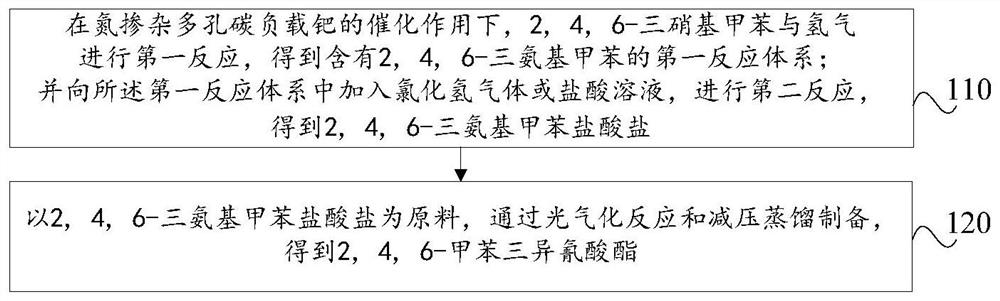
Preparation method of 2,4,6-toluene triisocyanate
A technology of toluene triisocyanate and trinitrotoluene, which is applied in the field of preparation of 2,4,6-toluene triisocyanate, can solve the problem of large-scale production of triaminotoluene and its hydrochloride, lack of preparation and purification of isocyanate solids , TTI preparation and purification methods are limited, etc., to achieve high-quality production, reduce preparation costs, and low prices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0073] The catalyst nitrogen-doped porous carbon supported palladium used in the above preparation method is prepared by the following steps:
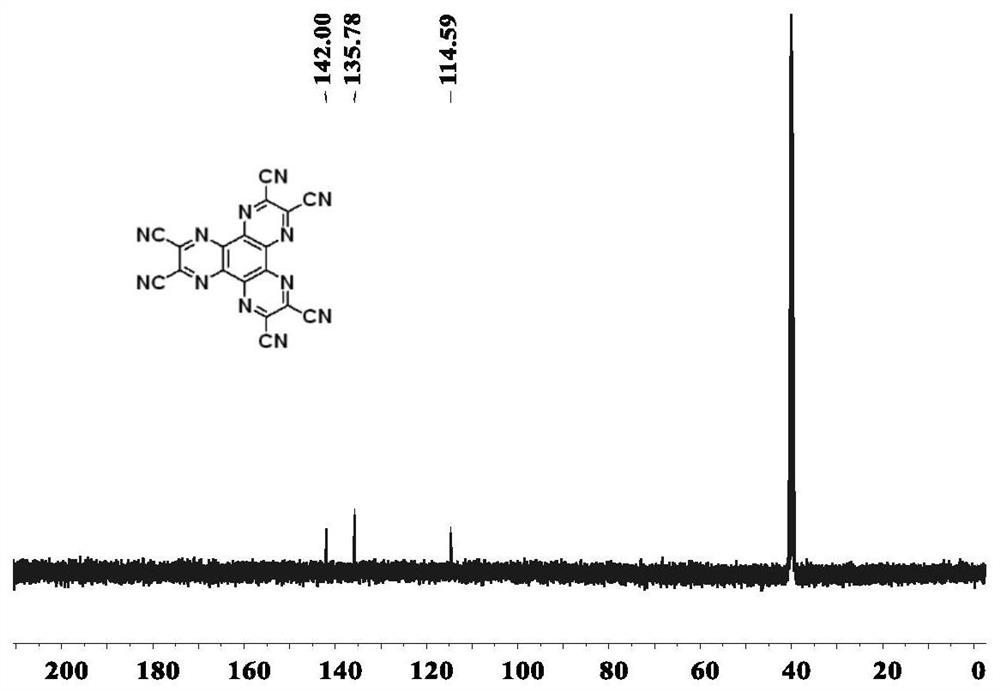
[0074] S21, step 1-1, using cyclohexanone shown in structural formula I and diaminomaleonitrile shown in structural formula II as raw materials, using a weak acid as a catalyst and solvent, and obtaining the hexacyanogen shown in structural formula III through the third reaction base pyrazine.
[0075] Specifically, using cyclohexanone shown in structural formula I and diaminomaleonitrile shown in structural formula II as raw materials, using acetic acid as a catalyst and solvent, the reaction temperature is 80°C to 140°C, and the reaction time is 1h to 10h Prepare the hexacyanopyrazine (HAT(CN) shown in structural formula III below 6 ).
[0076] S22, step 1-2, using the hexacyanopyrazine shown in the structural formula III as a raw material, through a cleavage reaction and a reduction reaction, to obtain the nitrogen-doped porous ca...
Embodiment approach
[0078] Strategy 1: using hexacyanopyrazine shown in structural formula III as a raw material, performing a cracking reaction to prepare nitrogen-doped porous carbon shown in structural formula IV; adding a palladium source and the nitrogen-doped porous carbon to the first solvent, performing a reaction to obtain palladium-supported nitrogen-doped porous carbon; the palladium-supported nitrogen-doped porous carbon undergoes a reduction reaction under the action of a first reducing agent to obtain the nitrogen-doped porous carbon-supported palladium.
[0079] Strategy 2: Add a palladium source and hexacyanopyrazine shown in structural formula III to the second solvent to react to obtain a mixture of palladium salt and hexacyanopyrazine shown in structural formula V; for the palladium salt and hexacyanopyrazine The mixture of hexacyanopyrazine is cleaved to obtain a cleavage product; the cleavage product undergoes a reduction reaction under the action of a second reducing agent to...
Embodiment 1
[0085] Step 1: HAT(CN) 6 Catalyst preparation.
[0086] Add cyclohexanone octahydrate (10g) and maleonitrile diamine (20g) into glacial acetic acid (400ml) and heat to reflux for 2h-3h, then filter while hot, and disperse the filter cake in about 30% nitric acid solution , heated at 80°C for 1h, washed with water, and dried to obtain brown solid HAT(CN) 6 (12g).
[0087] Step 2: Pd@C 2 Preparation of N catalyst.
[0088] The obtained tan solid HAT(CN) 6 (12g) was placed in a crucible, raised from room temperature to 700°C at a heating rate of 5°C / min, and kept at this temperature for 2 hours, and then lowered to room temperature at a cooling rate of 5°C / min to prepare a black sample C 2 N (10g).
[0089] Will C 2 N (10 g) and palladium acetate (0.2 g) were dispersed in deionized water (300 mL), sonicated for 10 min to disperse evenly, and excess sodium borohydride (1.0 g) aqueous solution was slowly added, and vigorously stirred for 60 min. Filtrate under reduced pres...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com