Alkaline system direct methanol fuel cell anode catalyst and preparation method thereof
A methanol fuel cell and catalyst technology, which is applied to fuel cells, battery electrodes, electrochemical generators, etc., can solve the problems of affecting the electrochemical reaction activity of methanol oxidation, complicated preparation process, and low uniform dispersion of active component nickel, etc. Achieving the effect of excellent electrochemical reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
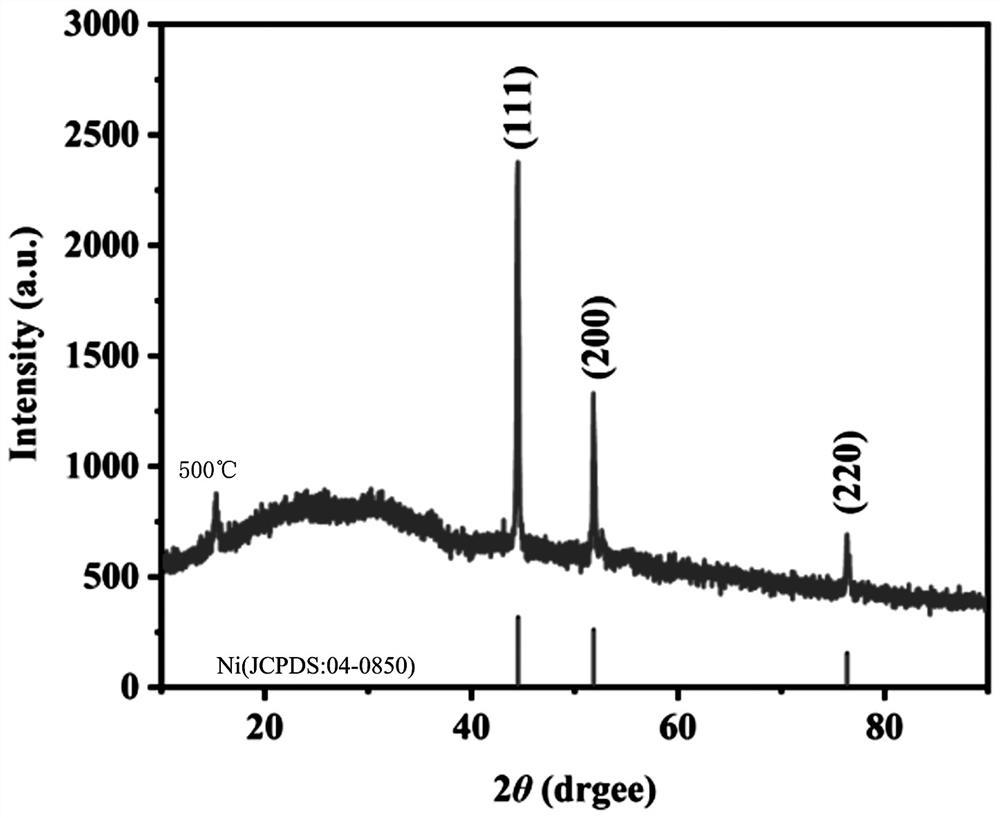
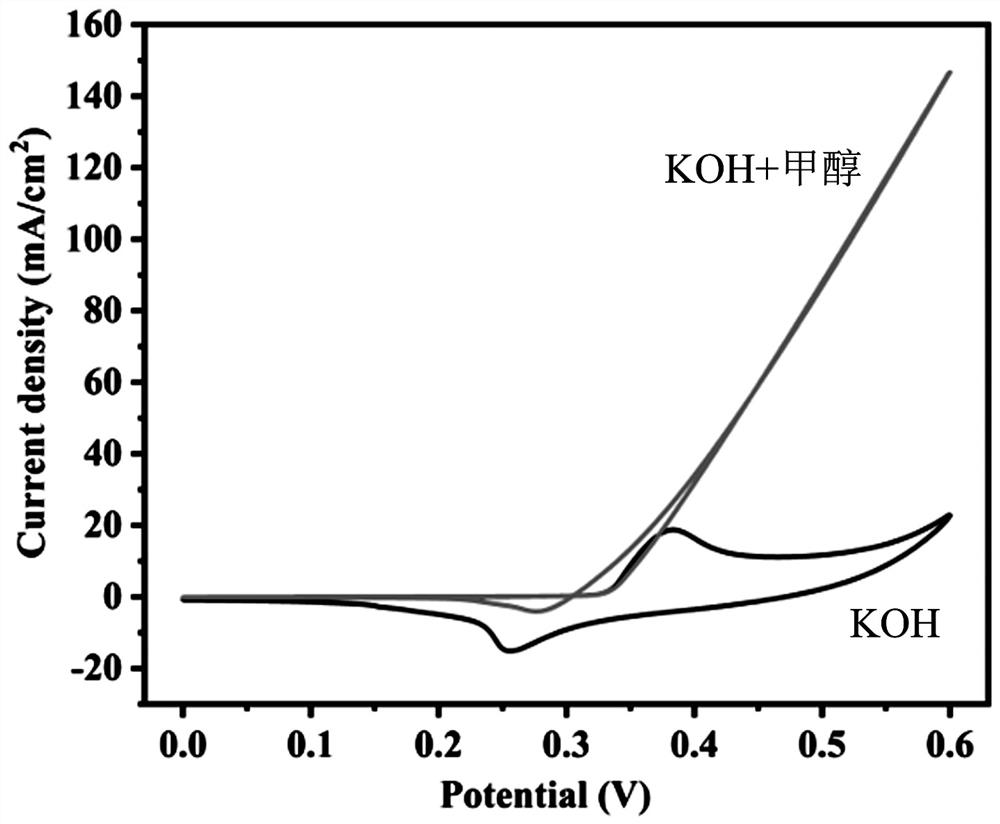
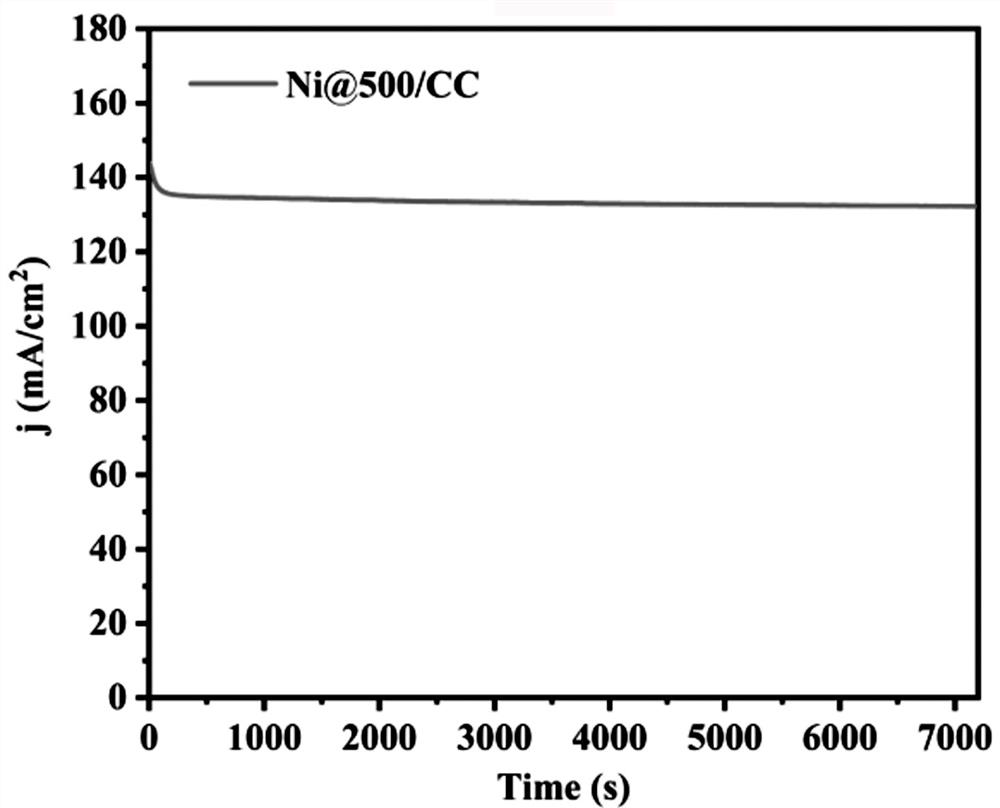
Image
Examples
Embodiment 1
[0028] Weigh 0.0364g (0.21mmol) of triaminophenyl borate hydrochloride (ABA), and dissolve it in 835µL of 6mol / L hydrochloric acid solution to prepare an ABA solution.
[0029] Add the above-mentioned ABA solution, 274µL (3mmol) aniline (AN) and 241µL deionized water to 2mL of 10wt% polyvinyl alcohol (PVA) solution in sequence, stir in a water bath at 55°C for 30min, until the solution is clear, place in an ice-water bath , cooled to 0°C, 1.65mL 2mol / L ammonium persulfate (APS) solution was added dropwise, stirred quickly and evenly, poured into the mold, and polymerized at room temperature for 12 hours to prepare polyvinyl alcohol-polyaniline conductive hydrogel polymer film.
[0030] After the formed polymer film is washed with deionized water, it is placed in a 5mol / L nickel chloride solution and soaked for 18h, taken out and washed with deionized water, freeze-dried for 10h, placed in a tubular reactor, and placed in a N 2 Under the atmosphere, the temperature was raised ...
Embodiment 2
[0034] Weigh 0.0242g (0.14mmol) of triaminophenyl borate hydrochloride (ABA), and dissolve it in 835µL of 6mol / L hydrochloric acid solution to prepare an ABA solution.
[0035] Add the above-mentioned ABA solution, 183µL (2mmol) aniline (AN) and 882µL deionized water to 2.5mL 8wt% polyvinyl alcohol (PVA) solution in sequence, stir for 30min under heating in a water bath at 55°C, until the solution is clear, place in an ice-water bath , cooled to 0°C, added dropwise 1.10mL 2mol / L ammonium persulfate (APS) solution, stirred quickly and evenly, poured into the mold, and polymerized at room temperature for 12 hours to prepare polyvinyl alcohol-polyaniline conductive hydrogel polymerized object film.
[0036] After the formed polymer film was washed with deionized water, it was placed in a 3mol / L nickel chloride solution and soaked for 18h, taken out and washed with deionized water, freeze-dried for 10h, placed in a tubular reactor, and placed in a N 2 Under the atmosphere, the te...
Embodiment 3
[0039] Weigh 0.0303g (0.175mmol) of triaminophenyl borate hydrochloride (ABA), and dissolve it in 835µL of 6mol / L hydrochloric acid solution to prepare an ABA solution.
[0040] Add the above-mentioned ABA solution, 229 µL (2.5 mmol) aniline (AN) and 561 µL deionized water to 2 mL of 12 wt% polyvinyl alcohol (PVA) solution in sequence, stir for 30 min under heating in a water bath at 55 °C, until the solution is clear, place in an ice-water bath , cooled to 0°C, added dropwise 1.38mL 2mol / L ammonium persulfate (APS) solution, stirred quickly and evenly, poured into the mold, and polymerized at room temperature for 12 hours to prepare polyvinyl alcohol-polyaniline conductive hydrogel polymerized object film.
[0041] After the formed polymer film is washed with deionized water, it is placed in a 5mol / L nickel chloride solution and soaked for 18h, taken out and washed with deionized water, freeze-dried for 10h, placed in a tubular reactor, and placed in a N 2 Under the atmosphe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com