Fluorescent probe based on tetraphenylethylene derivative as well as preparation method and application of fluorescent probe
A technology of tetraphenylethylene and fluorescent probe, which is applied in the field of fluorescence spectrum analysis, can solve the problems that the compound has not been researched and developed, and achieve the effect of low price, good stability and considerable yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
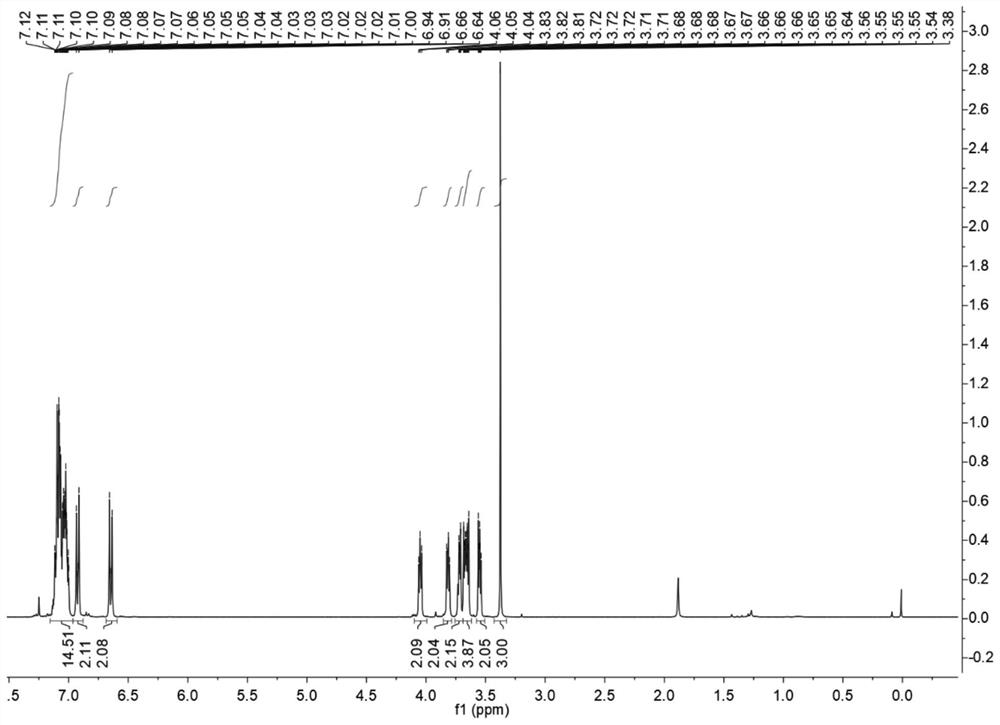
[0051] The synthesis of fluorescent probe 1 is carried out as follows:
[0052] (1) Weigh 3.2g of zinc powder (48mmol) and add it into a 100mL pressure bottle containing 50mL of anhydrous THF, and fill it with nitrogen for protection; then cool the mixture to 0°C, and slowly add 2.6mL of titanium tetrachloride ( 12mmol), the temperature was controlled below 10°C, the suspension mixture was heated to room temperature and stirred for 0.5h, then heated to reflux for 2.5h, then the mixture was cooled to 0°C, 0.5mL pyridine (6mmol) was added and stirred for 10 minutes, then weighed 0.88 g of benzophenone (4.8 mmol) and 0.81 g of 4-hydroxy-benzophenone (4.8 mmol) were added into 15 mL of anhydrous THF to dissolve, then added dropwise to the mixture, and refluxed for 10-12 hours. The reaction solution was then added to 25 mL of 10% K 2 CO 3 To quench the reaction, and extracted three times with dichloromethane, the organic layer was dried with anhydrous magnesium sulfate, filtered,...
Embodiment 2
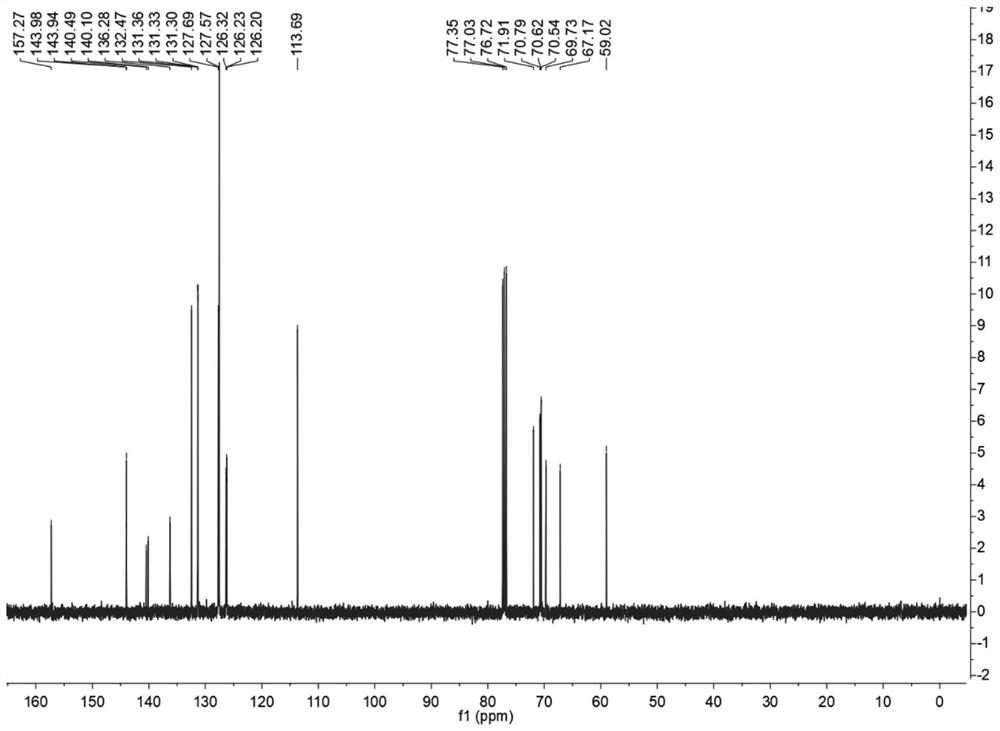
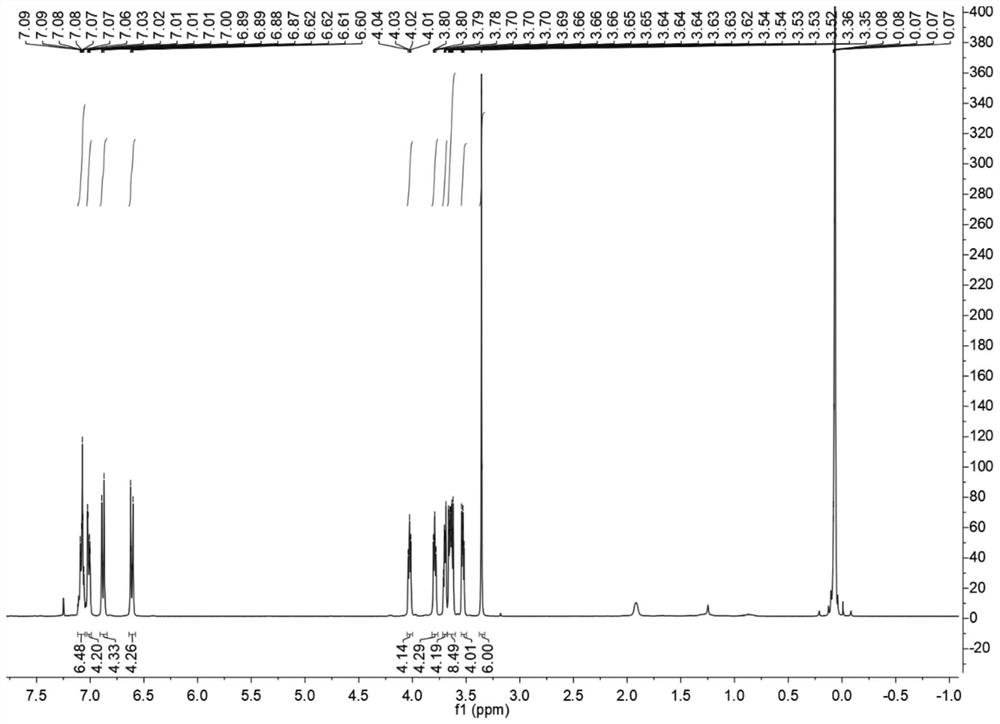
[0058] The synthesis of fluorescent probes 2 and 3 was carried out as follows:
[0059] (1) Weigh 1g of zinc powder (15.3mmol) and add it to a pressure bottle containing 25mL of anhydrous THF under nitrogen protection, cool the mixture to -5°C and add 1.1mL of TiCl dropwise with a syringe 4 , Refluxed at 75°C for 2h, then weighed 1.05g of 4-hydroxy-benzophenone (5mmol), dissolved it in 20mL of anhydrous THF, and slowly added it to the mixture, and refluxed at 75°C for 10-12h. The reaction solution was then added to 15 mL of 10% K 2 CO 3 To quench the reaction, and extract three times with dichloromethane, the organic layer was dried with anhydrous magnesium sulfate, filtered, the system was distilled under reduced pressure to spin dry the organic solvent, and the column chromatography [silica; ethyl acetate / petroleum ether; 1:4 (v / v)] isolated to obtain purified products, 1,2-bis(4-hydroxybenzene)-1,2-stilbene, 1,1-diphenyl-2,2-bis(4-hydroxyphenyl ) ethylene. Its reaction ...
Embodiment 3
[0066] Emission Spectra of Fluorescent Probes in Different Viscosity Systems
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com