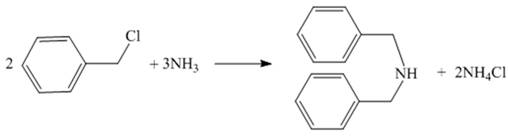
Preparation method of dibenzylamine
A technology of dibenzylamine and benzyl chloride, which is applied in the field of preparation of dibenzylamine, can solve the problems of high production raw material cost and difficult disposal of three wastes, and achieve the effects of reducing process safety hazards, less pollution, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1. Make sure that there is no water in the autoclave, and then use the Roots vacuum pump to pump the autoclave to the maximum vacuum (-0.1MPa).
[0030] 2. Pump 100g of liquid ammonia into the reaction kettle through a high-pressure pump, then raise the temperature to 55°C and keep it warm.
[0031] 3. Quantitatively beat 297g of benzyl chloride into the reaction kettle through a high-pressure pump, set the finishing time to 35 minutes, control the temperature at 50-60°C throughout the process, and stop feeding when the pressure is close to 2.5MPa, and cool down to 50-55°C .
[0032] 4. After the benzyl chloride has been fed, continue to keep warm and stir for 20 minutes, then release the pressure to release the excess liquid ammonia in the reaction kettle, and then seal the reaction kettle.
[0033] 5. Vacuumize to a slight negative pressure, put 302g of liquid caustic soda (32% sodium hydroxide solution) into the reaction kettle, continue stirring at 50-60°C for 20 m...
Embodiment 2
[0037] 1. Make sure that there is no water in the autoclave, and then use the Roots vacuum pump to pump the autoclave to the maximum vacuum (-0.1MPa).
[0038] 2. Pump 100g of liquid ammonia into the reaction kettle through a high-pressure pump, then raise the temperature to 50°C and keep it warm.
[0039] 3. Quantitatively beat 297g of benzyl chloride into the reaction kettle through a high-pressure pump, set the finishing time to 35 minutes, control the temperature at 50-60°C throughout the process, and stop feeding when the pressure is close to 2.5MPa, and cool down to 50-55°C .
[0040] 4. After the benzyl chloride has been fed, continue to keep warm and stir for 20 minutes, then release the pressure to release the excess liquid ammonia in the reaction kettle, and then seal the reaction kettle.
[0041] 5. Vacuumize to a slight negative pressure, put 302g of liquid caustic soda (32% sodium hydroxide solution) into the reaction kettle, continue stirring at 50-60°C for 20 m...
Embodiment 3
[0045] 1. Make sure that there is no water in the autoclave, and then use the Roots vacuum pump to pump the autoclave to the maximum vacuum (-0.1MPa).
[0046] 2. Pump 100g of liquid ammonia into the reaction kettle through a high-pressure pump, then raise the temperature to 58°C and keep it warm.
[0047] 3. Quantitatively beat 297g of benzyl chloride into the reaction kettle through a high-pressure pump, set the finishing time to 35 minutes, control the temperature at 50-60°C throughout the process, and stop feeding when the pressure is close to 2.5MPa, and cool down to 50-55°C .
[0048] 4. After the benzyl chloride has been fed, continue to keep warm and stir for 20 minutes, then release the pressure to release the excess liquid ammonia in the reaction kettle, and then seal the reaction kettle.
[0049] 5. Vacuumize to a slight negative pressure, put 302g of liquid caustic soda (32% sodium hydroxide solution) into the reaction kettle, continue to stir at 50-60°C for 20 mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com