Synthetic method of 2, 6-dichloro-4-((4-hydroxyphenyl)amino)phenol
A synthesis method and aminophenol technology are applied in the preparation of amino hydroxy compounds, chemical instruments and methods, preparation of organic compounds, etc., and can solve the problems of low reaction yield, long synthesis steps, and difficult purification, and achieve simple post-processing. , The effect of good product quality and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
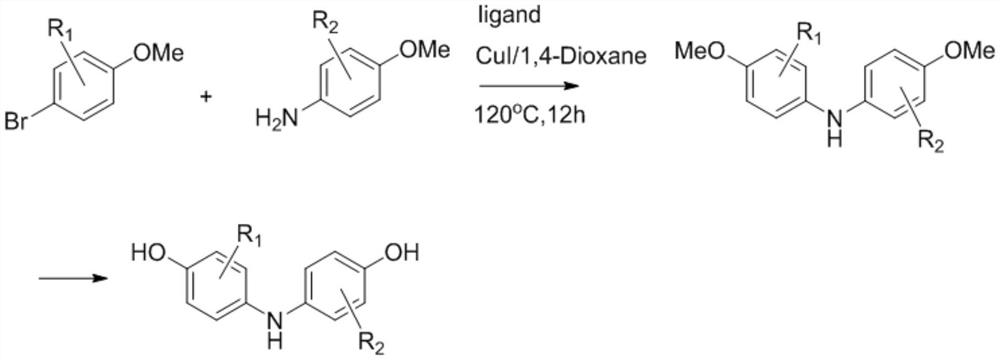
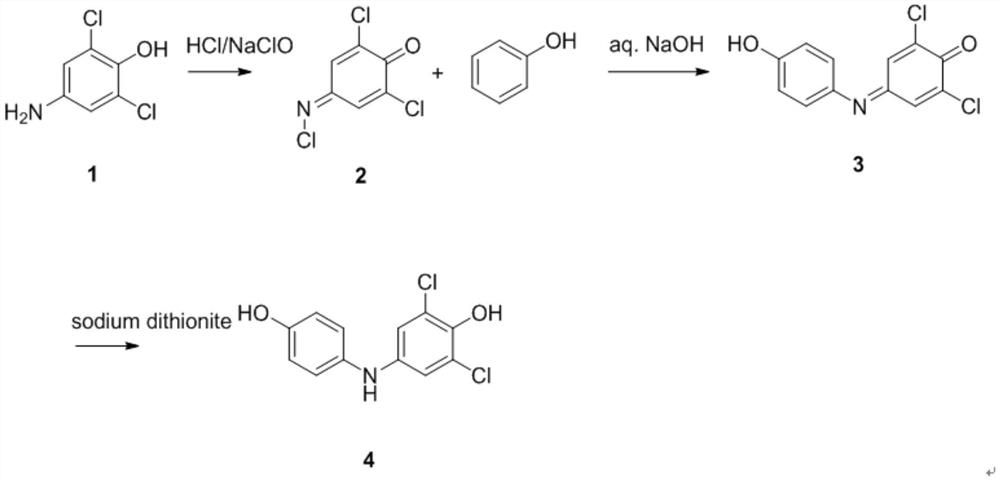
[0028] The invention discloses a synthesis method of 2,6-dichloro-4-((4-hydroxyphenyl)amino)phenol, which comprises the following steps:
[0029] Using 2,6-dichloro-4 aminophenol (1.0eq) and phenol (1.0eq) as raw materials, in the presence of 2N NaOH (1.5eq) solution and 6-14% sodium hypochlorite (1.5eq), make it with water 2,6-dichloro-4-((4-hydroxyphenyl)amino)phenol was synthesized in one step using solvent. The chemical reaction formula is as follows:
[0030]
[0031] The preparation method is as follows: 2,6-dichloro-4-aminophenol (1.0eq), phenol (1.0eq), 2N NaOH solution (1.5eq), 6-14% sodium hypochlorite (1.5eq), under nitrogen protection. During the reaction, 2,6-dichloro-4 aminophenol (1.0eq) and phenol (1.0eq) were dissolved in water, and a mixed solvent of 2N NaOH solution (1.5eq) and 6-14% sodium hypochlorite (1.5eq) was added dropwise. After the dropwise addition was completed, the reaction was continued to stir at room temperature for 3 hours.
[0032] The...
Embodiment 1
[0035] In a 250ml reaction flask, add 5.4g of 2,6-dichloro-4-((4-hydroxyphenyl)amino)phenol, 2.82g of phenol, 30ml of water, dropwise add 2N NaOH / 1M NaClO (22.5ml / 55ml ), keep the reaction temperature not higher than 35°C, after the dropwise addition is completed, continue to stir the reaction at room temperature for 3 hours, adjust the system to pH=8 with concentrated hydrochloric acid, filter, pass column purification, and elute with ethyl acetate / petroleum ether=10%, to obtain The target compound p-hydroxydiphenylamine was 5.76g, and the yield was 70.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com