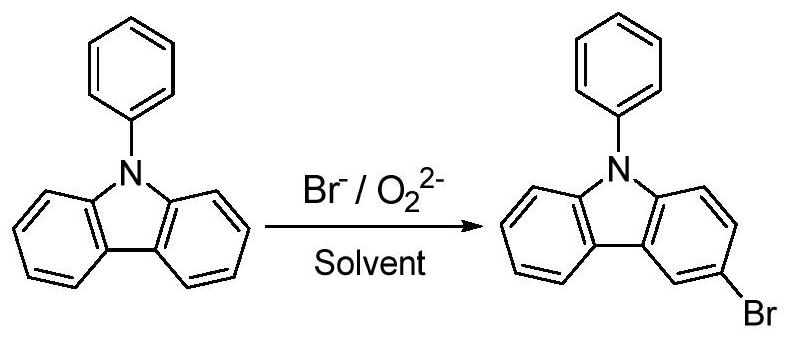
A method for synthesizing high-purity 3-bromo-n-phenylcarbazole
A phenylcarbazole and high-purity technology, which is applied in the field of organic synthesis of N-phenylcarbazole derivatives, can solve the problems of high reaction risk factor, complicated post-treatment process, difficult industrialized production and the like, and achieves easy control of the reaction. , easy to control the reaction drop rate, easy to control the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] At room temperature, add 24.3g of N-phenylcarbazole (99%, 0.10mol) and 50mL of dichloromethane into a 250mL four-necked round-bottomed flask equipped with a stirrer and a thermometer, stir until completely dissolved, and then reduce the reaction System temperature to -10°C; 24.3g hydrobromic acid (40%, 0.12mol, 17.6mL) was added dropwise, and 13.6g hydrogen peroxide (30%, 0.12mol, 12.2 mL), wherein, the dropping rate of the brominating agent is 3.0mL / min, the dropping rate of the oxidizing agent is 0.5mL / min, and the dropping process can strictly control the reaction temperature below -10°C; The reaction was carried out for 6 hours, during which the conversion rate of N-phenylcarbazole detected by the liquid phase reached ≥ 99%, and the reaction was stopped. After the reaction is finished, add quantitative sodium carbonate saturated aqueous solution to the reaction system to adjust the pH of the mixed solution to neutral, wash 3 times with water washing separation metho...
Embodiment 2
[0054] At room temperature, add 243.3g of N-phenylcarbazole (99%, 1.00mol) and 500mL of dichloromethane into a 1000mL four-necked round-bottomed flask equipped with a stirrer and a thermometer, stir until completely dissolved, and then reduce the reaction System temperature to -10°C; 212.4g hydrobromic acid (40%, 1.05mol, 153.9mL) was added dropwise, and 119.0g hydrogen peroxide (30%, 1.05mol, 107.2 mL), wherein, the dropping rate of the brominating agent is 3.0mL / min, the dropping rate of the oxidizing agent is 0.5mL / min, and the dropping process can strictly control the reaction temperature below -10°C; The reaction was carried out for 6 hours, during which the conversion rate of N-phenylcarbazole detected by the liquid phase reached ≥ 99%, and the reaction was stopped. After the reaction is finished, add quantitative sodium carbonate saturated aqueous solution to the reaction system to adjust the pH of the mixed solution to neutral, wash 3 times with water washing and separ...
Embodiment 3
[0056] This example is basically the same as Example 1, except that 40.5g of hydrobromic acid (20%, 0.10mol, 35.0mL) and 17.9g of hydrogen peroxide (20%, 0.105mol, 16.7mL) were added.
[0057] After the end of the reaction in this example, HPLC detected that the conversion rate of N-phenylcarbazole failed to reach 99%, and 24.5 g of 3-bromo-N-phenylcarbazole (the content detected by HPLC was ≥99%) had a yield of 76.0%; In the process of completing the reaction system of hydrobromic acid and hydrogen peroxide, the volume ratio of the inorganic phase to the organic phase is close to 1:1, and the concentration of bromine molecules generated is too low, so that N-phenylcarbazole cannot be completely converted into 3-bromo-N-phenyl Carbazole affects the conversion and yield of the reaction.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com