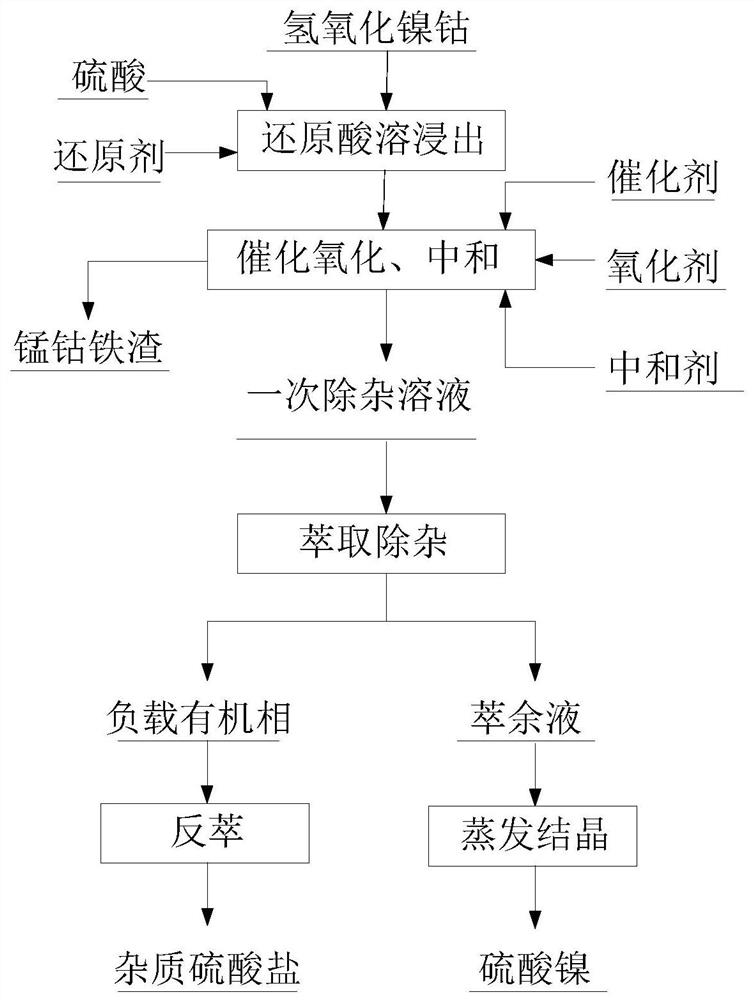
Method for preparing nickel sulfate from cobalt nickel hydroxide
A technology of nickel-cobalt hydroxide and nickel sulfate, applied in the field of hydrometallurgy, can solve the problems of large flow rate of nickel-cobalt separation system, extraction and impurity removal, complicated process flow, etc., so as to reduce equipment investment and occupation, reduce system and simplify preparation. The effect of the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] MHP nickel-cobalt hydroxide is used as the raw material, its water content is 60%, the dry basis contains 40% nickel, 3.5% cobalt and 6% manganese.
[0043] Atmospheric pressure leaching is adopted, the temperature is 80°C, the leaching time is 2h, the pH is controlled at 2.0, the liquid-solid ratio is 8:1, and the amount of sulfur dioxide introduced is 0.8 times the molar amount of manganese in the system.
[0044] The nickel leaching rate is 99.8%, the cobalt leaching rate is 99.8%, and the manganese leaching rate is 95%.
[0045] Afterwards, pure oxygen is introduced as an oxidant, sodium metabisulfite is added as a catalyst, wherein the catalyst accounts for 5% of the dry weight of the raw material, nickel carbonate is used as a neutralizer, the reaction temperature is 60°C, the leaching time is 10h, the pH is controlled at 4.0, and the liquid-solid ratio is 8: 1.
[0046] The cobalt precipitation rate is 99.9%, and the manganese precipitation rate is 99.5%. The ni...
Embodiment 2
[0050] MHP nickel-cobalt hydroxide is used as the raw material, its water content is 60%, the dry basis contains 40% nickel, 3.5% cobalt and 6% manganese.
[0051] Using 0.4MPa pressure leaching, the temperature is 150°C, the leaching time is 2h, the pH is controlled at 2.0, the liquid-solid ratio is 8:1, and sulfur dioxide is introduced, which is 0.8 times the molar amount of manganese in the system.
[0052] The nickel leaching rate is 99.9%, the cobalt leaching rate is 99.9%, and the manganese leaching rate is 99%.
[0053] Afterwards, pure oxygen is introduced as an oxidant, sodium metabisulfite is added as a catalyst, wherein the catalyst accounts for 5% of the dry weight of the raw material, nickel carbonate is used as a neutralizer, the reaction temperature is 60°C, the leaching time is 10h, the pH is controlled at 4.0, and the liquid-solid ratio is 8: 1.
[0054] The cobalt precipitation rate is 99.9%, and the manganese precipitation rate is 99.5%. The nickel sulfate ...
Embodiment 3
[0058] MHP nickel-cobalt hydroxide is used as the raw material, its water content is 60%, the dry basis contains 40% nickel, 3.5% cobalt and 6% manganese.
[0059] Atmospheric pressure leaching is adopted, the temperature is 80°C, the leaching time is 0.5h, the pH is controlled at 2.0, the liquid-solid ratio is 8:1, and sulfur dioxide is introduced, which is 0.8 times the molar amount of manganese in the system.
[0060] The nickel leaching rate is 99.5%, the cobalt leaching rate is 99.2%, and the manganese leaching rate is 91%.
[0061] Afterwards, pure oxygen is introduced as an oxidant, sodium metabisulfite is added as a catalyst, wherein the catalyst accounts for 5% of the dry weight of the raw material, nickel carbonate is used as a neutralizer, the reaction temperature is 60°C, the leaching time is 10h, the pH is controlled at 4.0, and the liquid-solid ratio is 8: 1.
[0062] The cobalt precipitation rate is 99.9%, and the manganese precipitation rate is 99.5%. The nick...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com