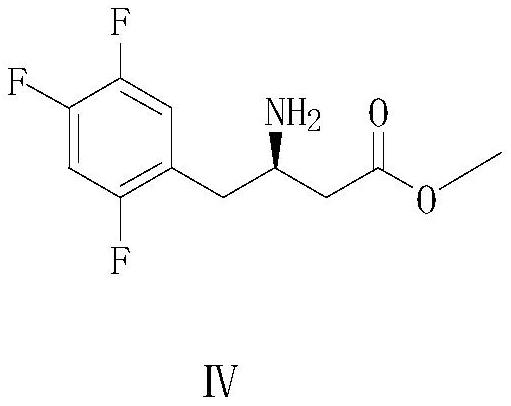
A kind of preparation method of (r)-3-amino-4-(2,4,5-trifluorophenyl) butyric acid methyl ester
A technology of trifluorophenyl and methyl butyrate, applied in the preparation of organic compounds, organic chemical methods, preparation of cyanide reactions, etc., can solve the problems of long reaction route, poor chiral selectivity of products, etc., and achieve good coordination ability, high chiral stereoselectivity, and the effect of improving catalytic reduction ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
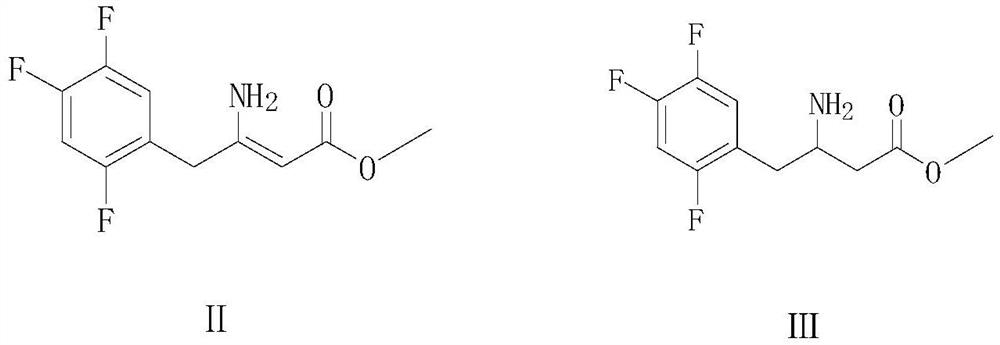
[0036] Preparation of 3-amino-4-(2,4,5-trifluorophenyl)but-2-enoic acid methyl ester(Ⅱ)
[0037] Add 80g of methanol and 20g of 3-oxo-4-(2,4,5-trifluorophenyl) methyl butyrate to a clean reactor, and then stir at room temperature for 10 minutes, then add 25.9g of Ammonium formate, then, slowly heat up to reflux and keep warm for 5 hours, TLC detection confirms that the reaction is complete, after the reaction, cool down to 50°C and control the temperature not to exceed 50°C, carry out vacuum distillation until no liquid flows out to remove the solvent, and obtain the corresponding concentrate , Then, add 60g of toluene and 30g of water to the concentrate, stir for 30 minutes, leave to stand for layering, collect the organic layer toluene layer, the water layer can be extracted once with 30g toluene, then combine the toluene layer, and add to the collected toluene layer Add 10g of anhydrous sodium sulfate, stir and dry at room temperature for 30 minutes, and filter with suction t...
Embodiment 2
[0046] Preparation of 3-amino-4-(2,4,5-trifluorophenyl)but-2-enoic acid methyl ester(Ⅱ)
[0047] Add 100 g of methanol and 20 g of 3-oxo-4-(2,4,5-trifluorophenyl) methyl butyrate to a clean reactor, then stir at room temperature for 10 minutes, add 23.3 g of formic acid Ammonium, heat up to reflux and keep it warm for 6 hours, TLC detection confirms that the reaction is complete, after the reaction, cool down to 50°C and control the temperature not to exceed 50°C, carry out vacuum distillation until no liquid flows out to remove the solvent, and obtain the corresponding intermediate product concentrate , Then, add 60g of toluene and 30g of water to the concentrate again, stir for 30 minutes, let stand to separate layers, collect the organic layer toluene layer, the water layer can be extracted once with 30g toluene, then merge the collected toluene layer, and pour it into the toluene layer Add 10g of anhydrous sodium sulfate, stir and dry at room temperature for 30 minutes, an...
Embodiment 3
[0056] Preparation of 3-amino-4-(2,4,5-trifluorophenyl)but-2-enoic acid methyl ester(Ⅱ)
[0057] Add 90g of methanol and 20g of 3-oxo-4-(2,4,5-trifluorophenyl) methyl butyrate (I) into a clean reactor, stir at room temperature for 15 minutes, add 24.5g Ammonium formate, then slowly warming up to reflux for 4 hours, TLC detection confirmed the completion of the reaction, after the reaction, cooled to 50 ℃ and under the condition that the temperature was controlled not to exceed 50 ℃, vacuum distillation was carried out until no liquid flowed out to obtain the corresponding Concentrate, then, add 60g toluene and 30g water again in the concentrate, stir 30 minutes, leave standstill layering, collect the organic layer toluene layer, the aqueous layer can be extracted once with 30g toluene, then merge the toluene layer, to the toluene layer collected Add 10g of anhydrous sodium sulfate, stir and dry at room temperature for 30 minutes, and filter with suction to obtain 3-amino-4-(2,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com