Probe capable of reducing radioactive kidney concentration based on enzyme digestion principle and preparation method thereof
A radioactive and radioactive labeling technology, applied in the field of medicine, can solve the problems of difficult prevention, restricting the dose of treatment, the number of courses of treatment and the final curative effect, and difficult to monitor sensitively, and achieves reduction of radioactive concentration and retention and excellent imaging. or therapeutic effect, the effect of high clinical promotion value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0074] As shown in the above synthetic route, the preparation method specifically includes the following steps:
[0075] 1) Mix the protected lysine with 2-chlorotriphenyl chloride resin at a molar ratio of 1.2:1, and connect the protected lysine under the action of N,N-diisopropylethylamine (DIPEA) onto the resin;
[0076] 2) On the basis of the product obtained in step 1), continue to connect amino acid X and Boc-Met to obtain the second-step product;
[0077] 3) The second-step product obtained in step 2) is deprotected by the protecting agent Dde under the action of 2% hydrazine hydrate to obtain the third-step product;
[0078] 4) The third step product obtained in step 3) is cut off from the resin under the action of 20% 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) to obtain the fourth step product;
[0079] 5) The fourth-step product obtained in step 4) is reacted with 3-(maleimido) propionic acid N-hydroxysuccinimide ester under the action of triethylamine to obtain the...
Embodiment 1
[0100] Prepare a class of exendin 4 compounds containing enzyme-cleaved sequences, and its synthetic route is as follows:
[0101]
[0102]
[0103] Concrete preparation process comprises the following steps:
[0104] 1. Synthesis of Compound 2:
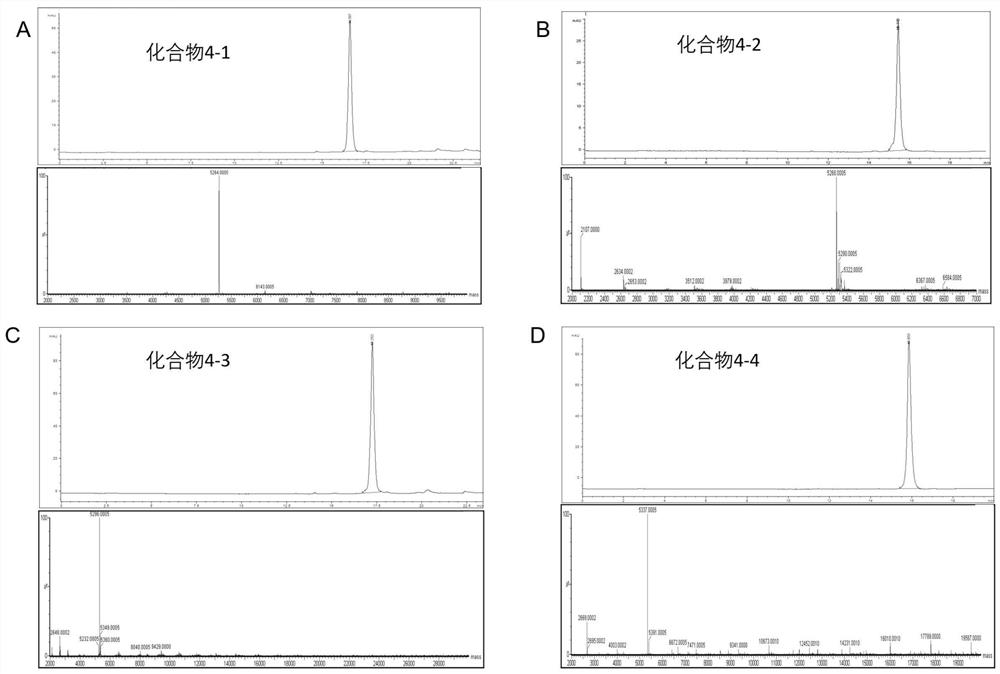
[0105] Add 0.5g Fmoc-Lys(Dde)-Resin (the amount of substance of Fmoc-Lys(Dde)-OH is 0.4mmol, 1 equivalent) in solid-phase synthesizer, take DMF as reaction solvent, with 0.8M DIPEA / DMF and 0.4M HBTU / DMF were used as activators, and 20% piperidine in DMF was used as the eluent of Fmoc. According to the sequence of the polypeptide, the second amino acid (Fmoc-Gly-OH, Fmoc-Val-OH , Fmoc-Phe-OH or Fmoc-Trp-OH, 4 equivalents) and Boc-Met-OH (0.399 g, 4 equivalents) to grow the peptide chain. After the solid-phase synthesis, the crude polypeptide product attached to the resin was placed in 3 mL of 2% hydrazine hydrate in DMF, stirred at room temperature for 1.5 h, filtered with suction, and the resin was washed with dichloromethan...
Embodiment 2
[0112] Preparation of a class of Z containing enzyme cleavage sequences HER2:2891 The compound, its synthetic route is as follows:
[0113]
[0114] Concrete preparation process comprises the following steps:
[0115] 1. The synthesis of compounds 1-2, 1-3, 2-2, 2-3, 3-2 and 3-3 is the same as in Example 1;
[0116] 2. Synthesis of Compound 5:
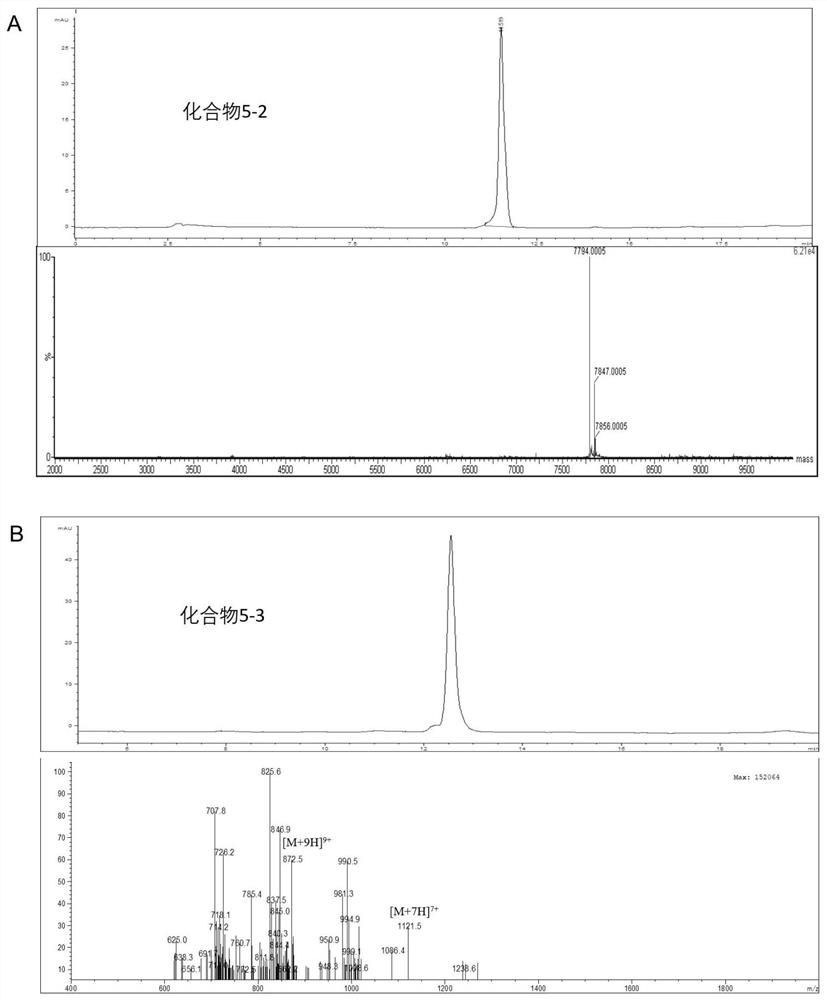
[0117] Compound 3 (0.66 μmol, 1.5 equiv) was added to Z HER2:2891 (commercially available, 3 mg, 0.44 μmol, 1 equiv.) in PBS solution, reacted at room temperature for two hours, and separated and purified by HPLC to obtain compound 5 with a yield of about 54-62%. After identification by LC-MS, the molecular weights of compounds 5-2 and 5-3 are [M+H] + =7794.00 and [M+7H] 7+ =1121.50, the calculated value (m / z) is 7793.90 (C 343 h 543 N 93 o 106 S 4 ) and 7841.90 (C 347 h 543 N 93 o 106 S 4 ). The HPLC analysis diagrams and LC-MS diagrams of compounds 5-2 and 5-3 are shown in figure 2 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com