Synthetic method of ginkgo leaf polyprenol metal complex with light-operated activity
A technology of ginkgo biloba polyprenol and metal complexes, applied in the direction of organic active ingredients, pharmaceutical formulas, medical preparations of non-active ingredients, etc., to achieve the effect of good dark stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
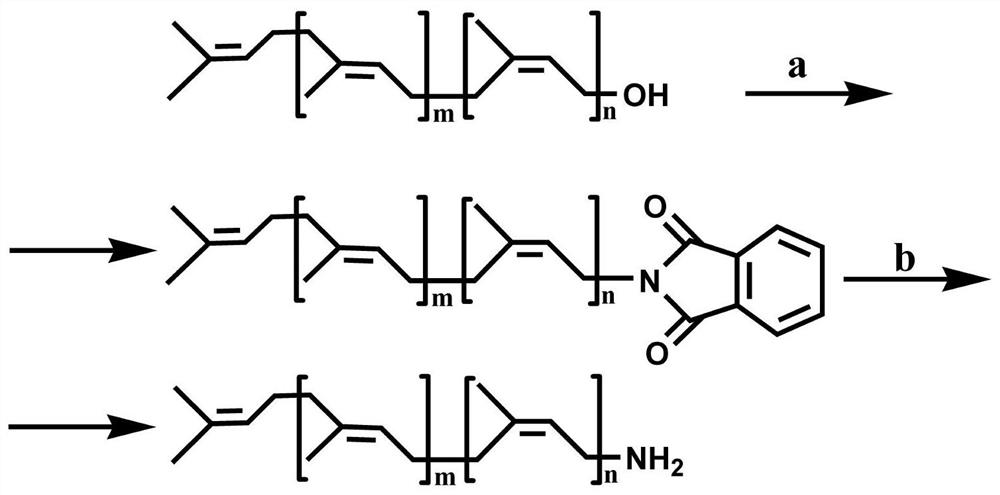
[0040] The synthetic route of aminopolypentenol is as figure 1 shown.
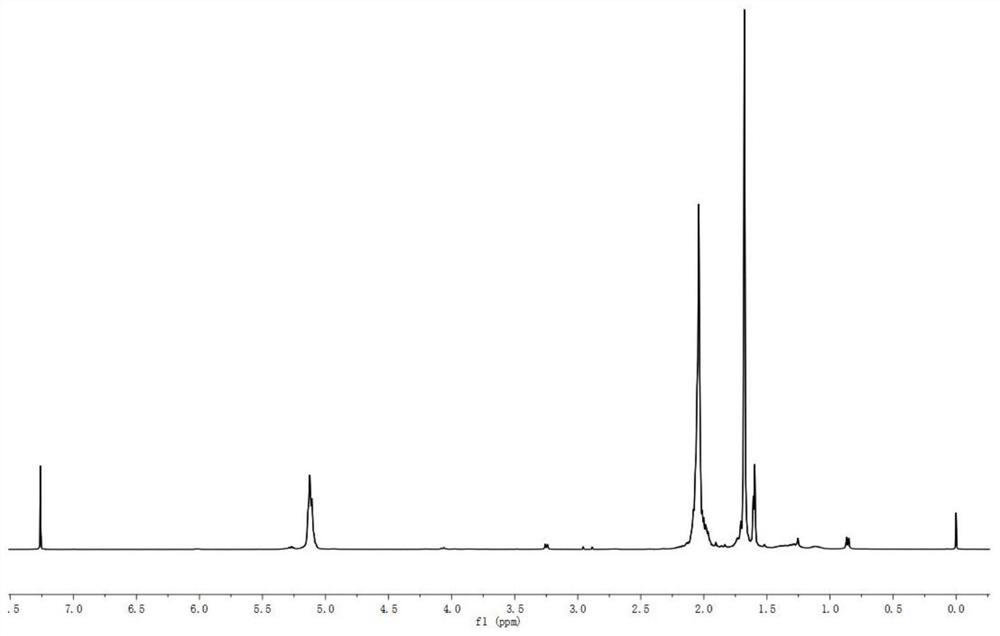
[0041] Weigh 0.27g of polyprenol, 0.06g of phthalimide and 0.11g of triphenylphosphine in a 35mL thick-walled pressure-resistant bottle, add 2.5mL of tetrahydrofuran, and then add 0.08g of diethyl azodicarboxylate Ester, reacted with magnetic stirring at 20°C for 4.0h (nitrogen atmosphere), concentrated after the reaction, the concentrate was dissolved in ether, after standing for a period of time, filtered to remove ether-insoluble substances, and concentrated ether solution to obtain 0.44g concentrate A, Concentrate A is light yellow oil, dissolve Concentrate A with a small amount of ether and then put it on a silica gel column (silica gel is 200-300 mesh, silica gel column is 2cm×40cm, 10g) for elution, eluent is ether: n-hexane Alkane=1:4, eluate A (0.21g) was light yellow oil, its Rf was 0.6 (developing solvent was diethyl ether:n-hexane=1:4);
[0042] Weigh 0.44g of eluate A and 0.24g of hydrazine ...
Embodiment 2
[0045] Weigh 30 mg of aminopolyprenol ligand, 11.3 mg of potassium hydroxide in 5 mL of water-ethanol (1:9) mixed solution, stir with a collector type constant temperature heating magnetic stirrer for 30 min, then add 100 mg of cis-Ru ( bpy) 2 Cl 2 , Reflux 2h. The solvent was removed by rotary evaporation to obtain 0.17 g of the concentrate. Then use a silica gel column for separation. Weigh 6g of silica gel, the silica gel column is 2cm×40cm, use 200-300 mesh silica gel, and rinse with eluent (chloroform-methanol=100:4). The collected compounds were dissolved in ethanol and NH 4 PF 6 Solid precipitated, filtered with filter paper to obtain a red precipitate, washed with water, and dried.
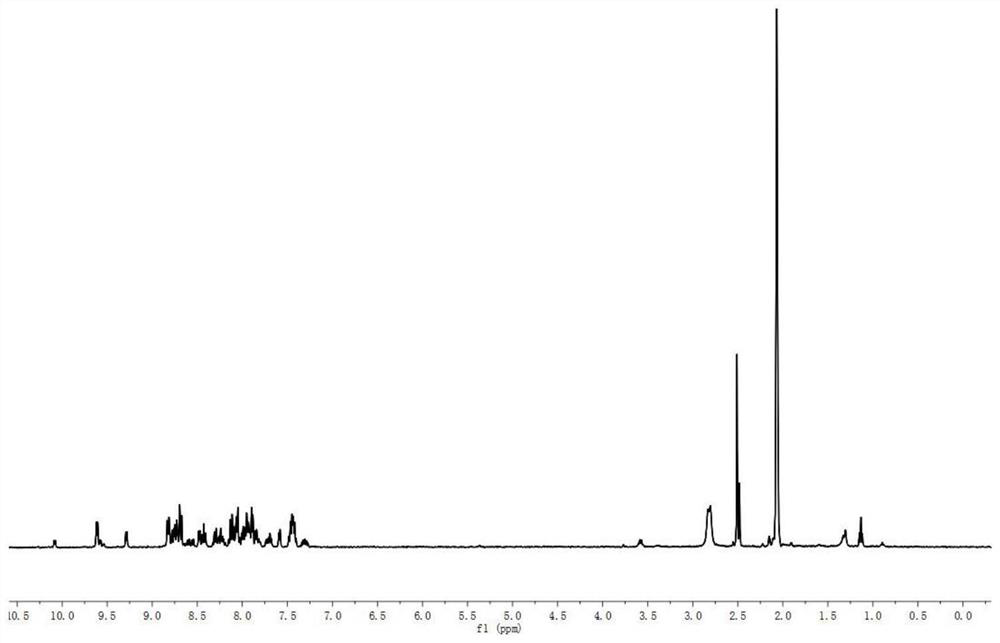
[0046] The hydrogen spectrum shift of aminopolyprenol metal complexes (such as image 3 shown) is parsed in two parts:
[0047] (1) Aminopolyprenol moiety 1 H NMR, δ: 5.27 (br-t,=C H -CH 2 -N); 5.12(m,=CH); 3.26(br-d,N-CH 2 ); 1.90~2.08(m,-CH 2 ); 1.71 (br-s,α-cis-CH 3 ); 1.6...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com