Preparation of bio-based aromatic monomer and preparation method of related homopolyester and copolyester
A technology of aromatic monomer and homopolyester, which is applied in the field of green synthesis, can solve the problems of restricting the application of renewable resources, etc., and achieve the effect of green synthesis method, novel monomer, and favorable industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
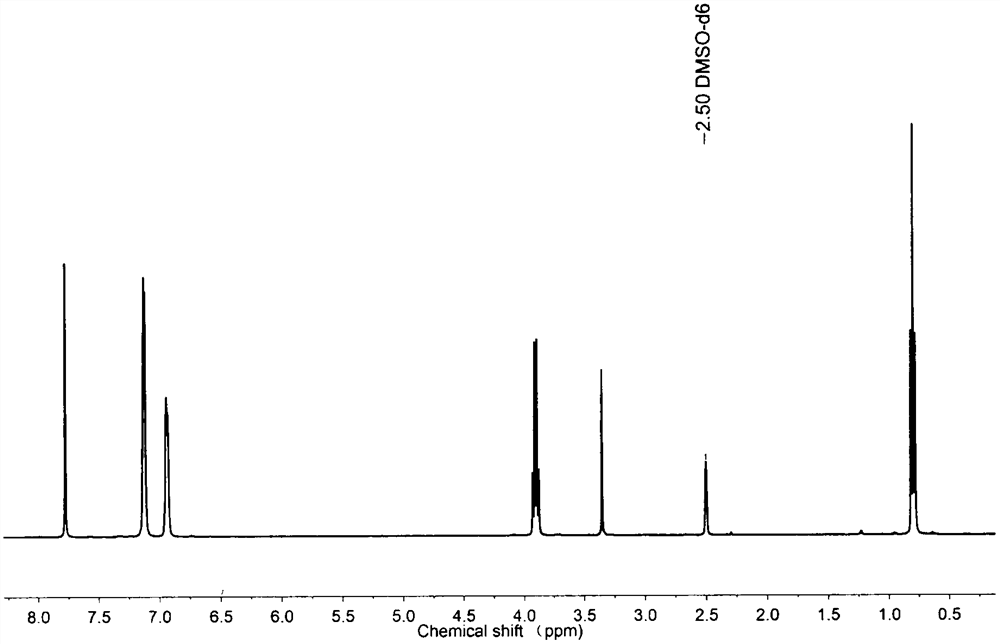
[0031] Embodiment 1: Preparation of 2,3-diphenyl-1,4-diethyl terephthalate monomer
[0032] After benzil (10g, 0.05mol), diethyl 1,3-acetonedicarboxylate (12g, 0.06mol) and methanol (70mL) were mixed and dissolved, sodium hydroxide (10g) was added and stirred at room temperature for 24h. The resulting product 2,5-diethoxycarbonyl-4-hydroxyl-3,4-diphenylcyclopentadienone (50g, 0.13mol) and acetic anhydride (100mL) were mixed uniformly, and then diluted with acetic anhydride was added dropwise Concentrated sulfuric acid (7 mL) solution was stirred at room temperature for 12 h, and quenched by adding water (300 mL). The resulting product 2,5-diethoxycarbonyl-3,4-diphenylcyclopentadienone (20g, 0.05mol) was mixed with dicycloheptadiene (19g, 0.21mol) and toluene (100mL) at 110 It was heated to reflux at ℃ for 12h, the solvent was removed under reduced pressure, and white crystals were obtained by recrystallization with methanol.
Embodiment 2
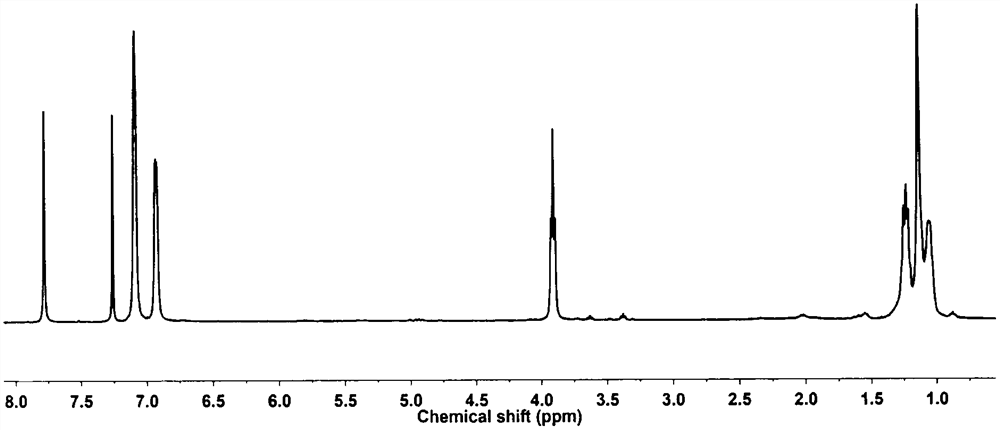
[0033] Example 2: Synthesis of homopolyester by 2,3-diphenyl-1,4-diethyl terephthalate and 1,10-decanediol
[0034] Add 2g (5.34mmol) 2,3-diphenyl-1,4-diethyl terephthalate and 1.49g (8.55mmol) 1,10-decanediol into a 100mL well-sealed three-neck flask, add Catalyst antimony trioxide and zinc acetate 0.63mg (2.67×10 -3 mmol), slowly feed nitrogen and continue to stir, heat to 160°C, react at this temperature for 3h, then raise the temperature to 240°C for 4h, remove the nitrogen protection, change the vacuum to 3mmHg, heat up to 260°C and continue the reaction for 8h , to obtain a viscous reaction solution, after the reaction solution was cooled to room temperature, 10 mL of chloroform was added to dissolve it, and then 100 mL of precipitating agent methanol was added to precipitate a white solid, which was filtered by suction and fully dried to obtain a homopolyester.
Embodiment 3
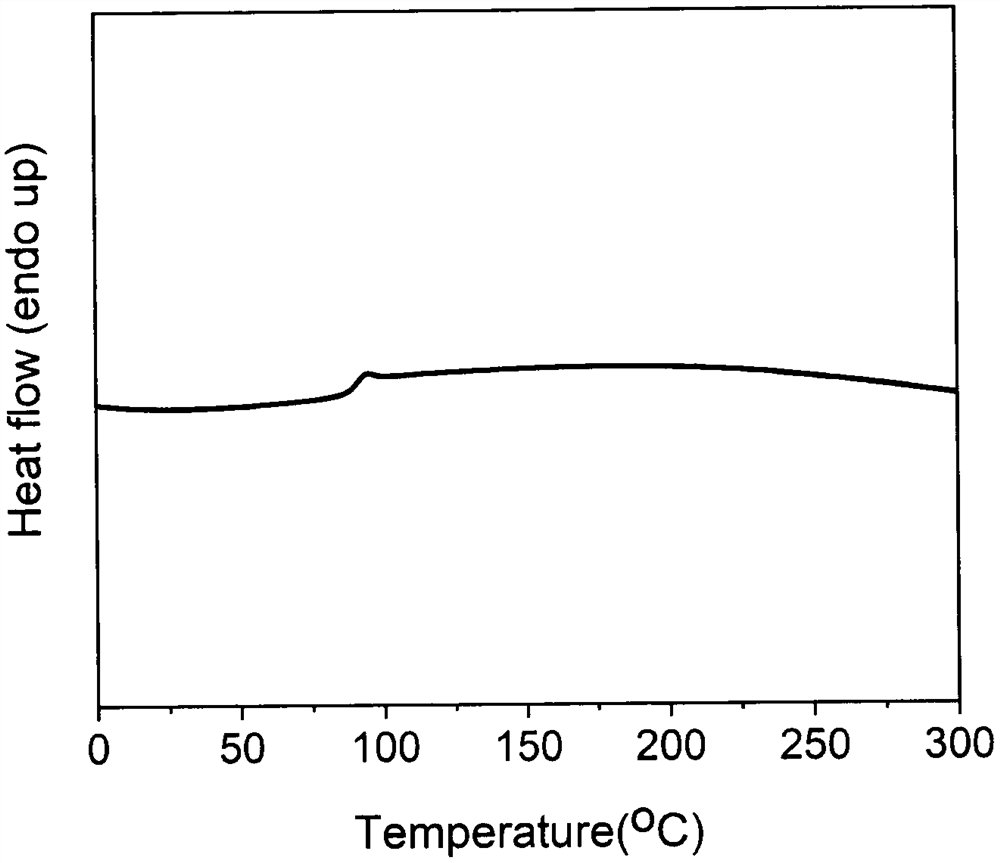
[0035] Example 3: Synthesis of homopolyester by 2,3-diphenyl-1,4-diethyl terephthalate and 1,8-octanediol
[0036]Add 2g (5.34mmol) 2,3-diphenyl-1,4-diethyl terephthalate and 1.25g (8.55mmol) 1,8-octanediol into a 100mL well-sealed three-neck flask, add Catalyst antimony trioxide and zinc acetate 0.63mg (2.67×10 -3 mmol), slowly feed nitrogen and continue to stir, heat to 160°C, react at this temperature for 3h, then raise the temperature to 240°C for 4h, remove the nitrogen protection, change the vacuum to 3mmHg, heat up to 260°C and continue the reaction for 8h , to obtain a viscous reaction solution, after the reaction solution was cooled to room temperature, 10 mL of chloroform was added to dissolve it, and then 100 mL of precipitating agent methanol was added to precipitate a white solid, which was filtered by suction and fully dried to obtain a homopolyester. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com