Phosphate ionic liquid and preparation method of phosphate ionic liquid, application and extraction method
An ionic liquid and phosphate ester technology, applied in the field of lithium extraction, can solve the problems of ionic liquid loss, low extraction efficiency, etc., and achieve the effects of reducing the use ratio, improving extraction performance, and solving low extraction efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
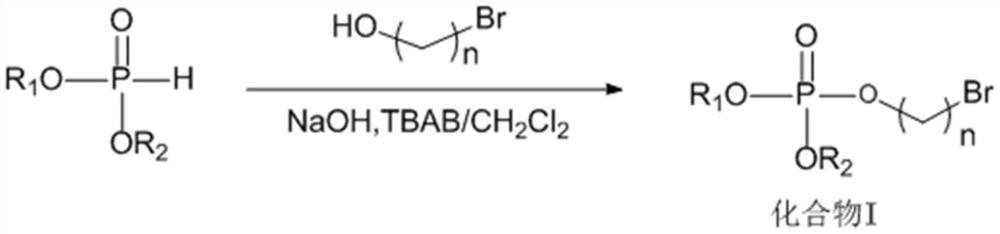
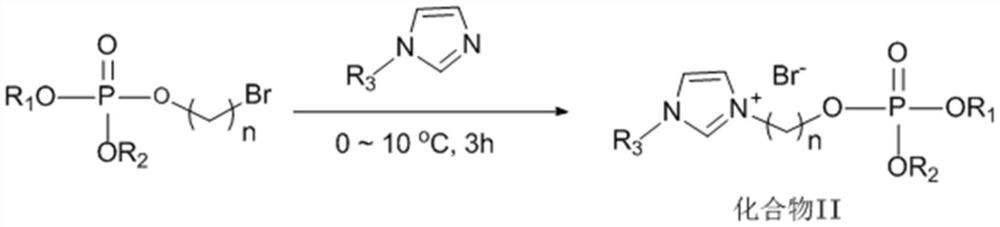
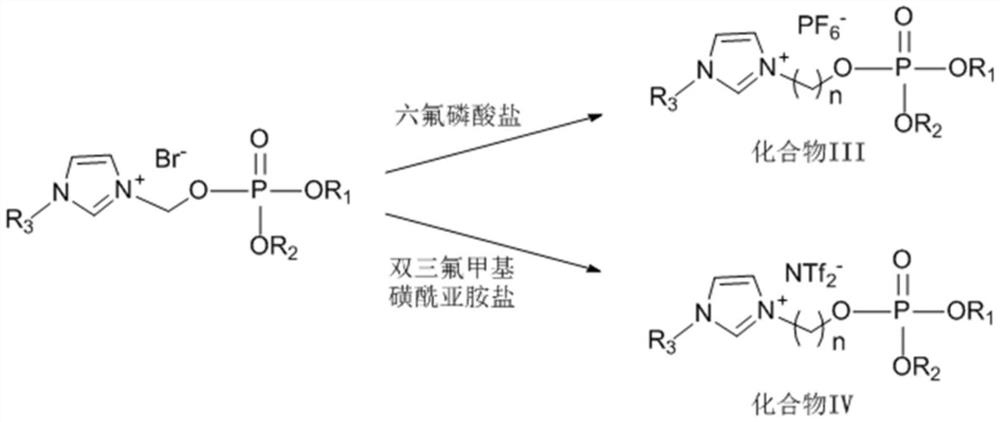
[0032]A phosphate ionic liquid, the preparation method thereof includes the following steps:
[0033](1) Into a four-necked flask equipped with a stirrer, a constant pressure dropping funnel, a thermometer, and a gas tube, sequentially add 25wt% sodium hydroxide aqueous solution (35mL), 3-bromopropanol (20.85g), carbon tetrachloride (30mL), dichloromethane (30mL), and tetrabutylammonium bromide (TBAB, 0.39g). Combine di-n-butyl phosphite (36.38g) with carbon tetrachloride at 10-15°C in an ice-water bath. The mixed solution (35mL) was slowly dropped into the above system through a constant pressure dropping funnel, the ice-water bath was removed, and the reaction was carried out at room temperature for 4h, diluted and filtered with dichloromethane (25mL), and then successively used 2vt% dilute hydrochloric acid (25mL) Washed 3 times, deionized water (25mL) washed 3 times, and then dried over anhydrous sodium sulfate, carbon tetrachloride and dichloromethane were removed under reduced pr...
Embodiment 2
[0044]A phosphate ionic liquid, the preparation method thereof includes the following steps:
[0045](1) Into a four-necked flask equipped with a stirrer, a constant pressure dropping funnel, a thermometer, and a gas tube, sequentially add 25wt% sodium hydroxide aqueous solution (35mL), 4-bromobutanol (22.95g), carbon tetrachloride (30mL), dichloromethane (30mL) and tetrabutylammonium bromide (TBAB, 0.39g), and diisoamyl phosphate (44.44g) and carbon tetrachloride (35mL The mixed solution of) was slowly dropped into the above system through a constant pressure dropping funnel, the ice-water bath was removed, and the reaction was carried out at room temperature for 4 hours, diluted and filtered with dichloromethane (25mL), and then washed with 2vt% dilute hydrochloric acid (25mL) 3 After washing three times with deionized water (25 mL), drying with anhydrous sodium sulfate, removing carbon tetrachloride and dichloromethane under reduced pressure, to obtain compound I (phosphoric acid-br...
Embodiment 3
[0056]A phosphate ionic liquid, the preparation method thereof includes the following steps:
[0057](1) Into a four-necked flask equipped with a stirrer, a constant pressure dropping funnel, a thermometer, and a gas tube, sequentially add 25wt% sodium hydroxide aqueous solution (35mL), 4-bromobutanol (22.95g), carbon tetrachloride (30mL), dichloromethane (30mL) and tetrabutylammonium bromide (TBAB, 0.39g), and dibutyl phosphite (36.38g) and carbon tetrachloride (35mL The mixed solution of) was slowly dropped into the above system through a constant pressure dropping funnel, the ice-water bath was removed and the reaction was carried out at room temperature for 4 hours, diluted and filtered with dichloromethane (25mL), and then washed with 2vt% dilute hydrochloric acid (25mL) 3 After washing three times with deionized water (25 mL), drying with anhydrous sodium sulfate, removing carbon tetrachloride and dichloromethane under reduced pressure, to obtain compound I (phosphoric acid-bromo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com