Acylphosphine photoinitiator and preparation method thereof
A technology of photoinitiator and acylphosphine, which is applied in the field of acylphosphine photoinitiator and its preparation, to achieve the effects of mild reaction conditions, strong light absorption ability and enhanced matching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The structural formula of 2,4,6-trimethylbenzoyl-diphenylphosphine oxide (TPO) is as follows
[0053]
[0054] In a dry three-necked flask (50 ml) equipped with a magnetic stirrer, a thermometer, a reflux condenser, and a nitrogen gas conduit, feed dry nitrogen to remove the air in the three-necked flask. Put 17.4g of aluminum trichloride, 17.9g of phenylphosphine dichloride, and 11g of benzene into a three-necked flask in sequence, and slowly raise the temperature of the oil bath to the set temperature of 90°C, and keep the reaction for 12h. After the unreacted raw material was evaporated under reduced pressure, it was cooled to room temperature; the reaction liquid was slowly added dropwise to 100 g of ice-water mixture for hydrolysis, and the reaction was continued for 4 hours after the hydrolysis was completed. 120 g of toluene was put into the reaction solution for extraction, and the organic layer was washed with alkali and water to obtain a toluene solution of...
Embodiment 2
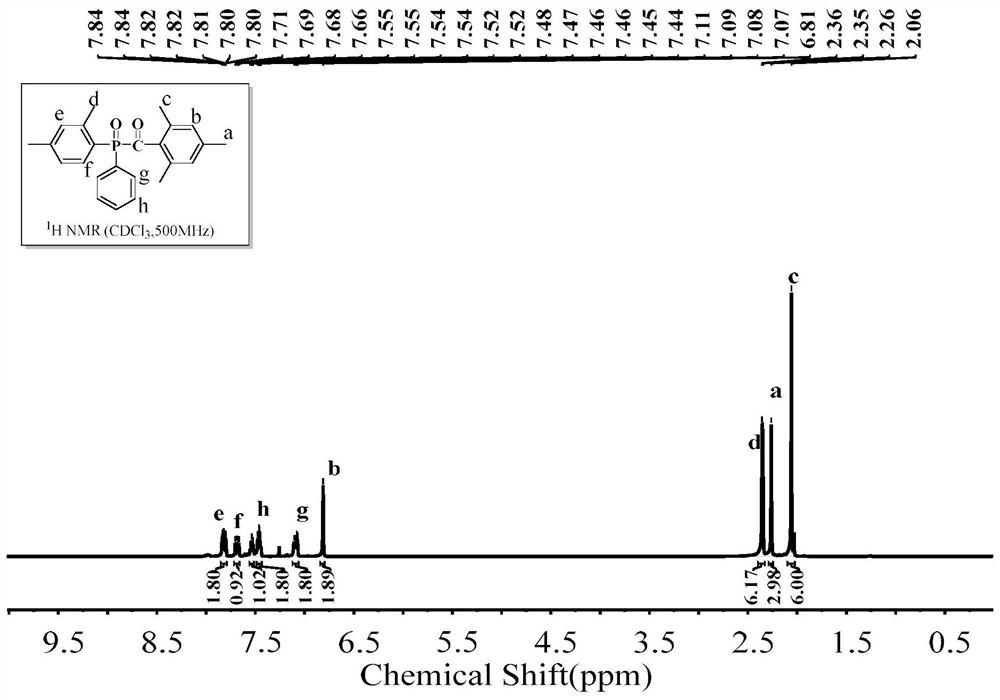
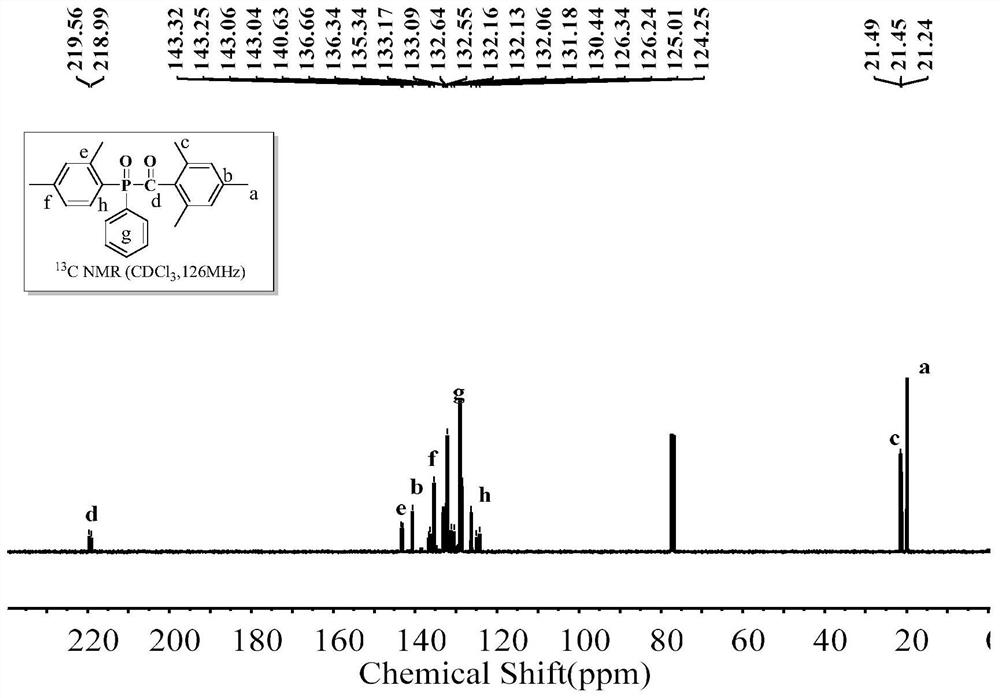
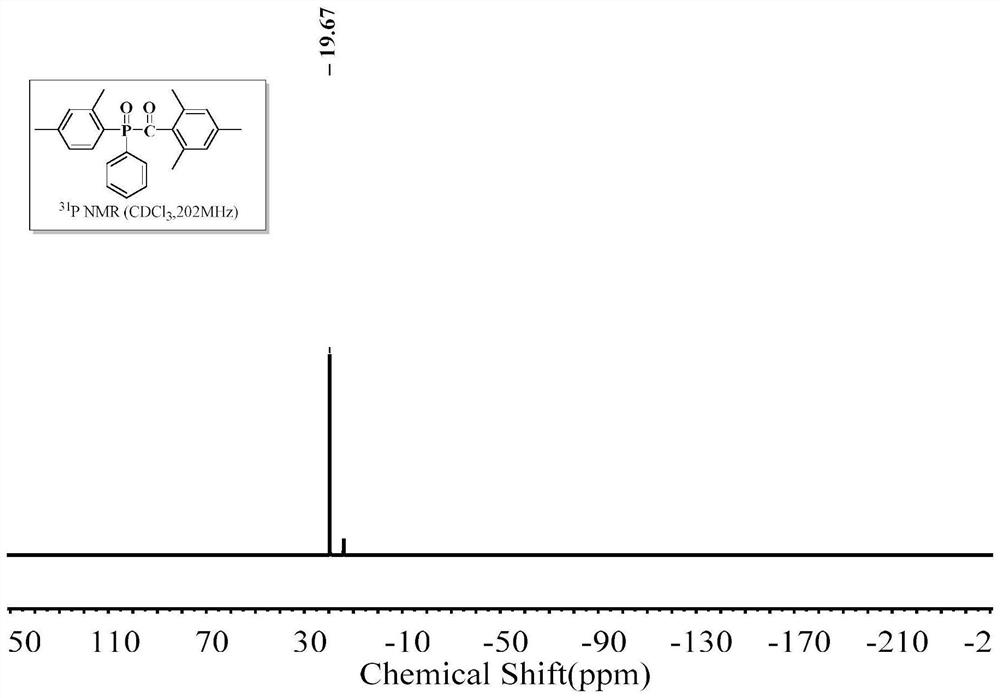
[0057] The structural formula of 2,4,6-trimethylbenzoyl-4-tolylphenylphosphine oxide (4-MTPO) is as follows:
[0058]
[0059] In a dry three-necked flask (50 ml) equipped with a magnetic stirrer, a thermometer, a reflux condenser, and a nitrogen gas conduit, feed dry nitrogen to remove the air in the three-necked flask. Put 17.4g of aluminum trichloride, 17.9g of phenylphosphine dichloride, and 12.9g of toluene into a three-necked flask in sequence, and slowly raise the temperature of the oil bath to the set temperature of 90°C, and keep the reaction for 12h. After the unreacted raw material was evaporated under reduced pressure, it was cooled to room temperature; the reaction liquid was slowly added dropwise to 100 g of ice-water mixture for hydrolysis, and the reaction was continued for 4 hours after the hydrolysis was completed. 120 g of toluene was put into the reaction solution for extraction, and the organic layer was washed with alkali and water to obtain a toluene ...
Embodiment 3
[0062] The structural formula of 2,4,6-trimethylbenzoyl-2,,4-xylylphenylphosphine oxide (2,4-DMTPO) is as follows:
[0063]
[0064] In a dry three-necked flask (50 ml) equipped with a magnetic stirrer, a thermometer, a reflux condenser, and a nitrogen gas conduit, feed dry nitrogen to remove the air in the three-necked flask. Put 17.4g of aluminum trichloride, 17.9g of phenylphosphine dichloride, and 14.9g of m-xylene into a three-necked flask in sequence, and slowly raise the temperature of the oil bath to the set temperature of 90°C, and keep the reaction for 12h. After the unreacted raw material was evaporated under reduced pressure, it was cooled to room temperature; the reaction liquid was slowly added dropwise to 100 g of ice-water mixture for hydrolysis, and the reaction was continued for 4 hours after the hydrolysis was completed. 120 g of toluene was put into the reaction solution for extraction, and the organic layer was washed with alkali and water to obtain a t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com