Iridoid glycoside compound and preparation method and application thereof
A technology of iridoid glycosides and compounds, applied in the field of medicine, can solve the problems of inconvenient storage and use, high price, etc., and achieve the effects of excellent anti-inflammatory effect, easy preparation, and easy control of process parameters
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
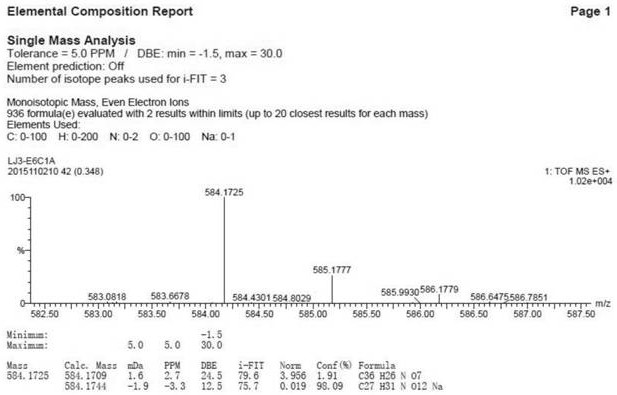
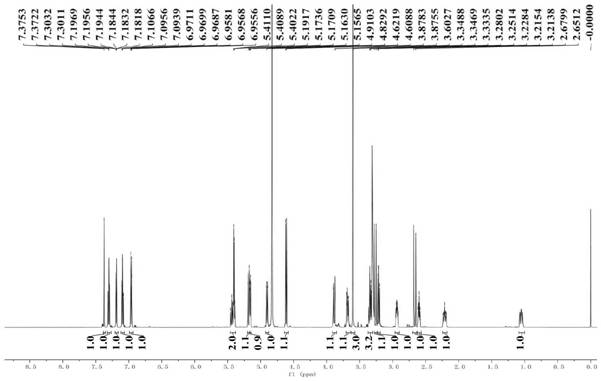
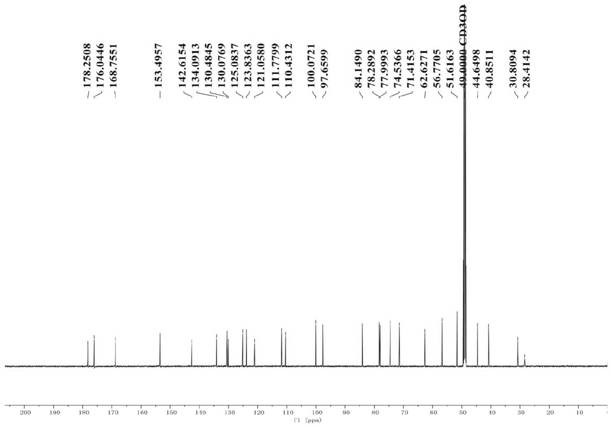
[0065]Example 1 Preparation and structure identification of iridoid glycosides
[0066]1. Instrument:
[0067]Nuclear magnetic resonance experiment: Swiss Bruker Avance 2600 fully digital superconducting nuclear magnetic resonance instrument; UV spectrum: JASCOV-550 ultraviolet-visible spectrometer; Infrared spectrum: JASCO FT / IR-480 plus infrared spectrometer, KBr tablet; ESI- MS: FINIGAN LCQ Advantage MAX mass spectrometer; HR-ESI-MS and UPLC-Q-TOF-MS: Waters Synapt G2 Q-TOF-MS; analytical high performance liquid chromatography (Waters): Waters e2695+DAD 2998; preparative High performance liquid chromatograph (Waters): Waters 1515+2489 UV / Visible; Column: C18 column (Phenomenex Gemini, 5μm, 4.6×250mm); C18column (Phenomenex Gemini, 5μm, 10×250mm); C18column (Waters, Aquity , BEH, 1.7μm, 3.0×150mm); Multifunctional microplate reader: Synergy HT (Bio-Tek Instruments Inc., Burleigh, USA); Thermo Scientific BB15 CO2 cell incubator, American Thermoelectric; Nikon TS100 inverted microscope; U...
Embodiment 2
[0084]Example 2 Test method for inhibitory activity of inflammatory factor IL-6:
[0085]1. Experimental process:
[0086]Compounds I-IV were prepared with DMSO to prepare a mother solution (100mM) of the required concentration, and 1.2 μL was added to 1199 μL of serum-free DMEM. After mixing, 495 μL was added to each well in a 24-well plate.
[0087]The Raw264.7 cells were digested with 0.25% trypsin (containing 0.02% EDTA), and the cell density was adjusted to 1×10 in DMEM medium containing 10% FBS.5Pieces / ml, evenly inoculated to a 24-well plate, 400μl per well. After planting the plate, put it in the incubator for 24 hours. After culturing for 24 hours, remove the 24-well plate, aspirate the supernatant, and add the drug-containing medium prepared by serum-free DMEM medium: ①Solvent control group: add 495μl of serum-free DMEM containing 1 / 1000 DMSO to each well ②Model group: Add 495μl of serum-free DMEM medium containing 1 / 1000 of DMSO to each well; ③Dosing sample group: Add 495μl of med...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com