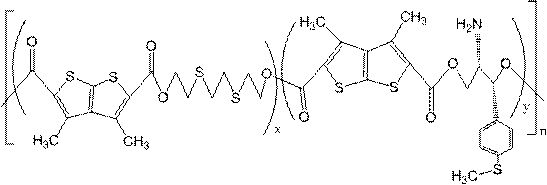
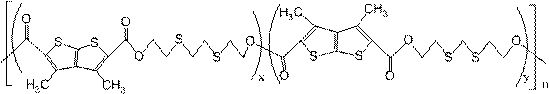
Preparation and application of sulfur-containing copolyester partially derived from biomass
A technology of sulfur-containing copolyester and biomass, which is applied in the preparation of sulfur-containing copolyester and the field of sulfur-containing copolyester, can solve the problems of poor thermal performance, mechanical performance, poor degradation performance, and poor mechanical performance, and achieve Easy to degrade, high molecular weight, good mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] To a dry single-necked flask (50 mL) were successively added 1.2815 g (5 mmol) of 3,4-dimethyl(2,3-B)thiophene-2,5-dicarboxylic acid (DTDA), 0.8414 g (5 mmol) of 3,5-Dithia-1,7-heptanediol (DTH), 0.9115g (5mmol) of 3,6-dithia-1,8-octanediol (DTO) and 0.0030g (0.35mmol) The germanium dioxide was obtained, and the reaction mixture was reacted under nitrogen protection at 150 °C for 5.0 h to obtain an esterified product. The temperature of the esterification product was continued to rise to 190° C., the vacuum degree in the reaction system was controlled at 40-100 Pa, and the crude product of the polycondensation product was obtained after the reaction for 2.0 h. After dissolving the crude product of the polycondensation product with a sufficient amount of chloroform, take the clear liquid, add the clear liquid to a certain amount of isopropanol, precipitate solid insolubles, centrifuge, and filter to obtain a white solid, and the obtained solid is treated with ethanol. T...
Embodiment 2
[0051] To a dry single-necked flask (50 mL), 1.2815 g (5 mmol) of 3,4-dimethyl(2,3-B)thiophene-2,5-dicarboxylic acid (DTDA), 1.066 g (5 mmol) of (1S,2S)-(+)-2-amino-1-[4-(methylthio)phenyl-1,3-propanediol, 0.948 g (5.2 mmol) of 3,6-dithia-1, 8-Octanediol (DTO) and 0.0026g (0.04mmol) of germanium dioxide, the reaction mixture was reacted under nitrogen protection at 155°C for 5.0h to obtain an esterified product. The temperature of the esterification product was continued to rise to 195° C., the vacuum degree in the reaction system was controlled at 40-100 Pa, and the crude product of the polycondensation product was obtained after the reaction for 3.0 h. After dissolving the crude product of the polycondensation product with a sufficient amount of chloroform, take the clear liquid, add the clear liquid to a certain amount of methanol, precipitate solid insolubles, centrifuge, and filter to obtain a white solid, and the obtained solid is washed with ethanol, The solid filtered...
Embodiment 3
[0054] To a dry single-necked flask (50 mL), 1.2815 g (5 mmol) of 3,4-dimethyl(2,3-B)thiophene-2,5-dicarboxylic acid (DTDA) and 0.8582 g (5.1 mmol) were sequentially added of 3,5-dithia-1,7-heptanediol (DTH), 0.9115g (5mmol) of 3,6-dithia-1,8-octanediol (DTO) and 0.0017g (0.005mmol ) of tetrabutoxide germanium, the reaction mixture was reacted at 150° C. under nitrogen protection for 5.0 h to obtain an esterified product. The temperature of the esterification product was continued to rise to 200° C., the vacuum degree in the reaction system was controlled at 40-100 Pa, and the crude product of the polycondensation product was obtained after the reaction for 2.0 h. After dissolving the crude product of the polycondensation product with a sufficient amount of chloroform, take the clear liquid, add the clear liquid to a certain amount of n-butanol, precipitate solid insolubles, centrifuge, and filter to obtain a white solid, and the obtained solid is treated with ethanol. The so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength at break | aaaaa | aaaaa |
| elongation at break | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com