Hydrogen evolution catalyst with nickel hydroxide layer coated elemental ruthenium structure and preparation method thereof
A technology of nickel hydroxide layer and nickel hydroxide, which is applied in the field of electrocatalysis, can solve the problem that the performance and stability of transition metal catalysts cannot be compared with platinum, and achieve good electrical conductivity and mechanical stability, safety and cost, and simple synthesis method Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Step 1, weigh a certain mass of ruthenium chloride hydrate, add it into 30 ml of 1M sodium hydroxide solution, and stir magnetically until uniform. The preparation concentration is 2.97×10 -4 mol / L ruthenium chloride solution.
[0031] In step 2, 0.2480 g of nickel foam sheet was weighed and washed with 3M hydrochloric acid, absolute ethanol and deionized water in sequence.
[0032] In step 3, the nickel foam is put into the uniformly stirred solution in step 1, placed in a homogeneous reactor, and reacted at 160° C. for 24 hours to obtain a catalyst with a nickel hydroxide layer coated with elemental ruthenium structure.
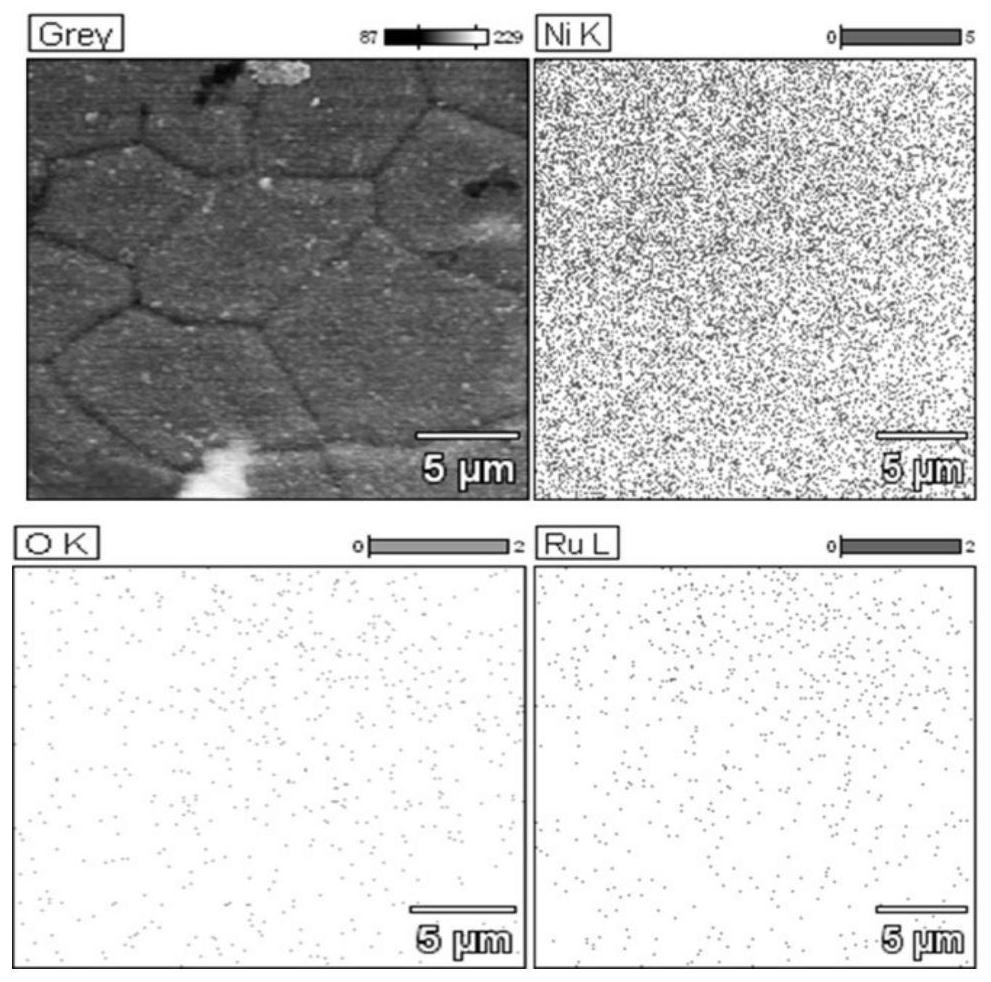
[0033] figure 1 It is a scanning electron microscope image of the catalyst with a nickel hydroxide layer coated with elemental ruthenium structure prepared by the one-step method in Example 1 of the present invention. It can be seen from the figure that the surface of the nickel foam is uniformly covered with a large number of wrinkled nanosheet s...
Embodiment 2
[0036] Step 1, weigh a certain mass of ruthenium chloride hydrate, add it into 30 ml of 1M sodium hydroxide solution, and stir magnetically until uniform. The preparation concentration is 5.95×10 -4 mol / L ruthenium chloride solution.
[0037]In step 2, 0.2480 g of nickel foam sheet was weighed and washed with 3M hydrochloric acid, absolute ethanol and deionized water in sequence.
[0038] In step 3, the nickel foam is put into the uniformly stirred solution in step 1, placed in a homogeneous reactor, and reacted at 160° C. for 24 hours to obtain a catalyst with a nickel hydroxide layer coated with elemental ruthenium structure.
[0039] Figure 7 The polarization curve diagram of the catalyst with the nickel hydroxide layer-coated elemental ruthenium structure prepared by the one-step method in Example 2 of the present invention. It can be seen from the figure that the catalyst prepared by this method can reach a current density of 10 mA cm -2 , the required overpotential ...
Embodiment 3
[0041] Step 1, weigh a certain mass of ruthenium chloride hydrate, add it into 30 ml of 1M sodium hydroxide solution, and stir magnetically until uniform. The preparation concentration is 2.97×10 -4 mol / L ruthenium chloride solution.
[0042] In step 2, 0.2480 g of nickel foam sheet was weighed and washed with 3M hydrochloric acid, absolute ethanol and deionized water in sequence.
[0043] In step 3, the foamed nickel is put into the uniformly stirred solution in step 1, placed in a homogeneous reactor, and reacted at 160° C. for 12 hours to obtain a catalyst with a nickel hydroxide layer coated with elemental ruthenium structure.
[0044] Figure 8 The polarization curve diagram of the catalyst with the nickel hydroxide layer-coated elemental ruthenium structure prepared by the one-step method in Example 3 of the present invention. It can be seen from the figure that the catalyst prepared by this method can reach a current density of 10 mA cm -2 , the required overpotenti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com