A kind of onoo-fluorescent probe, preparation method and application thereof
A fluorescent probe and structural formula technology, applied in the field of fluorescent probes, can solve problems such as inability to achieve large-scale synthesis, poor selectivity, complex chemical structure, etc., and achieve the effects of good popularization and application value, obvious colorimetric changes, and high sensitivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1. ONOO - Preparation of Fluorescent Probe L-1
[0039] 4-(N,N-Diethylamino)salicylaldehyde (0.193g, 1mmol) and 2-hydrazinepyridine (0.109g, 1mmol) were stirred in absolute ethanol (20ml) at 40°C for 24h to form a precipitate, and the mixture was extracted. Filtered and washed with absolute ethanol (10ml*3), and the product was dried to obtain a pale yellow solid with a yield of 55%.
[0040] Prepared ONOO - The fluorescent probes were characterized as follows: 1 H NMR (300MHz, CDCl 3 ): δ=10.73(s, 1H), 8.13(d, J=5.1Hz, 1H), 7.88(s, 1H), 7.71–7.60(m, 1H), 7.11–6.97(m, 2H), 6.79( dd,J=6.7,5.5Hz,1H),6.27(dq,J=4.9,2.5Hz,2H),3.41(q,J=7.1Hz,4H), 2.16(s,2H),1.22(t,J =7.1Hz,6H). 13 C NMR (75MHz, CDCl 3 ): δ=159.19, 155.91, 149.98, 147.17, 144.67, 138.60, 131.24, 115.34, 106.91, 106.62, 103.75, 98.27, 44.47, 12.66.LC-MS: m / z[M+H] + calcd for[C 16 H 20 N 4 O+H] + : 285.1710, found 285.1594.
[0041] Preparation of the ONOO - The reaction equation of the fluorescent...
Embodiment 2
[0052] Example 2 Study of UV Absorption and Fluorescence Spectra
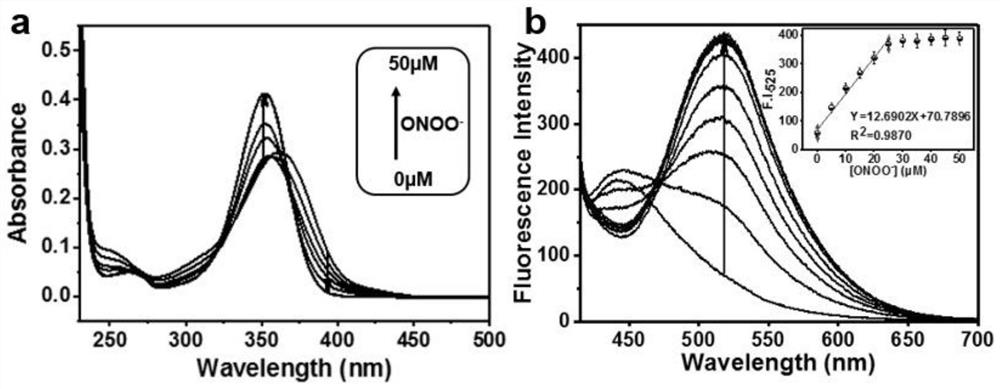
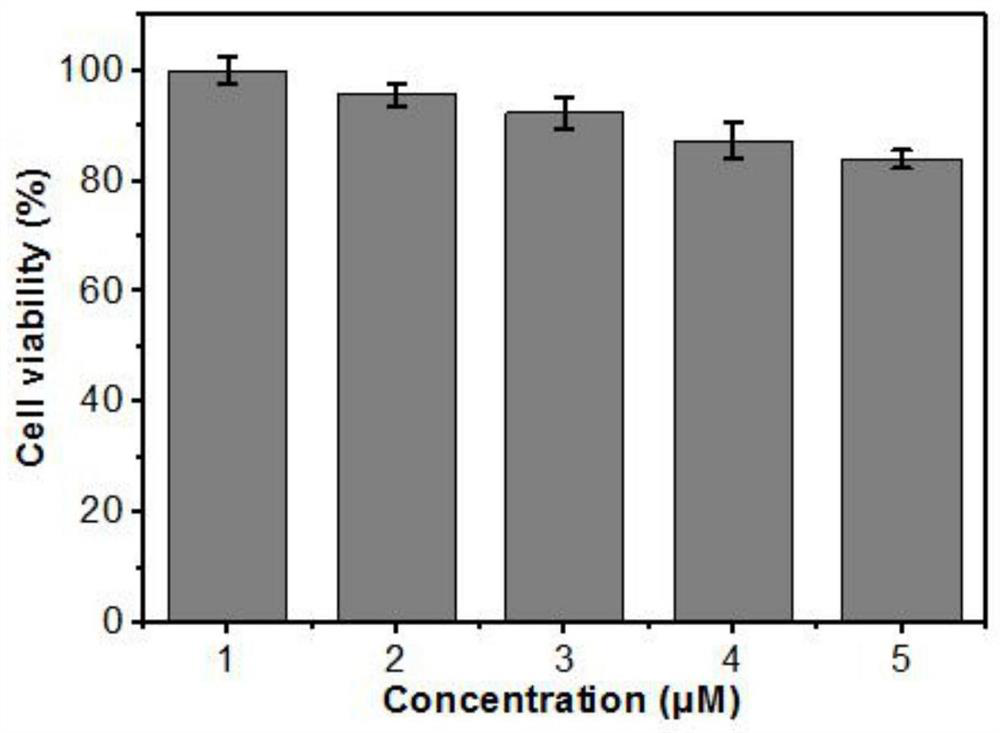
[0053] Monitoring Fluorescent Probe L-1 to Various Concentrations of ONOO by Titration Experiments - (0-50 μM) analytical power. in the absorption spectrum figure 1 a, with ONOO - The concentration of L-1 increased from 0 μM to 50 μM, the main absorption peak of the fluorescent probe L-1 showed a blue shift from 360 nm to 350 nm; figure 1 b, The weak fluorescence emission of the free probe at about 450 nm is weakened, a new emission band appears at 525 nm, and the fluorescence intensity increases accordingly. With ONOO - Increase in concentration at 0 to 25 μM ONOO - Concentration range, fluorescence intensity (525nm) and ONOO - There is an excellent linear relationship between the concentrations (R 2 =0.9870). When ONOO in solution - When the concentration is greater than 25 μM, the fluorescence intensity tends to be stable. The detection limit (S / N=3) of the fluorescent probe L-1 was determined to b...
Embodiment 3
[0054] Example 3 Effects of pH and reaction time on the response of fluorescent probe L-1
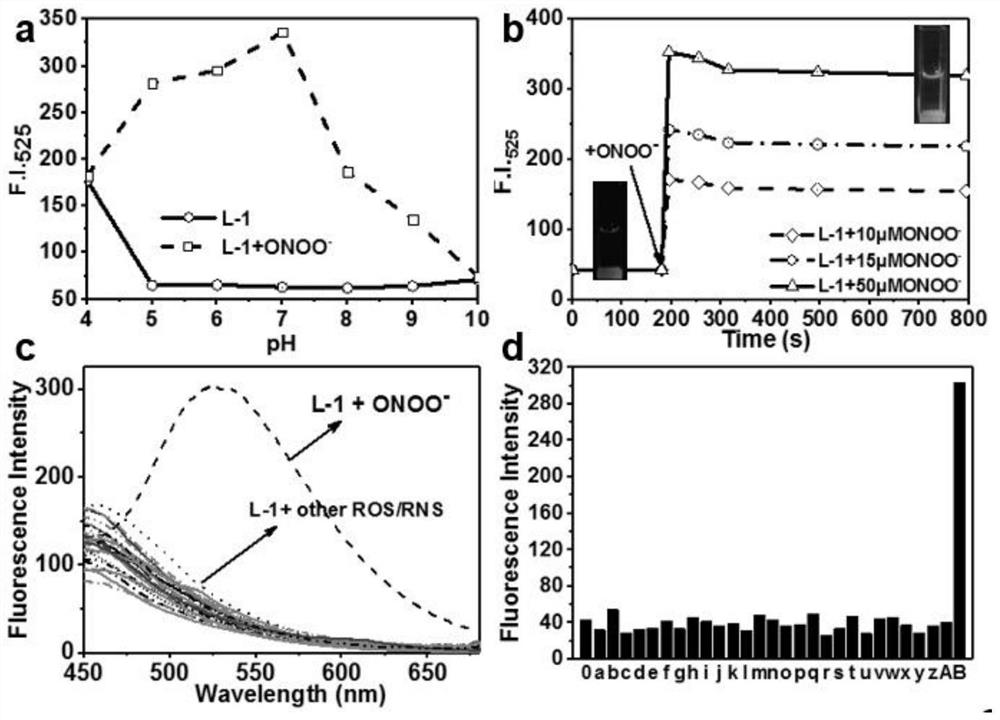
[0055] The fluorescent probe L-1 itself did not change significantly in a wide range (pH=5-10), which indicated that the pH value had almost no effect on the fluorescence emission of L-1. When the fluorescent probe L-1 and ONOO - In response, L-1 responds to ONOO - After the response, the fluorescence intensity reached the maximum value at pH=7, which indicated that the probe L-1 was sensitive to ONOO under neutral conditions. - The fluorescence change in response is the most pronounced. In order to simulate the in vivo detection environment, physiological pH (pH=7.4) was selected as the working pH in the following experiments. Afterwards, the response time, as an important factor to evaluate the actual sensing performance of the probe, was also investigated. like figure 2 a, in the absence of ONOO - In the case of , almost no fluorescence change of probe L-1 was observed. like ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com