Preparation method for removing aromatic methyl ether and methyl
A technique for removing aromatic methyl ether, applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of difficult recovery of methyl tertiary butyl ether, large amount of methyl tertiary butyl ether, average yield and purity, etc., and achieves the preparation method. Green, less by-products, high product yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
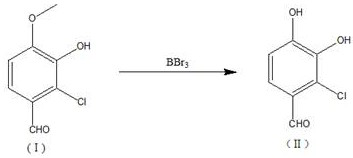
[0016] Add 2g (0.011mol) 2-chloro-4-methoxy-3-hydroxybenzaldehyde to a 100ml three-necked flask, add 8ml of dichloromethane, stir until dissolved, and drop 5g (0.019mol) of BBr at -30°C 3 , after the dropwise addition was completed, the reaction solution was placed at room temperature and stirred for 4 hours. Under an ice-water bath environment (-10°C), 25ml of methanol was slowly added dropwise. ) adjust the pH of the reaction solution to 3~4, heat to reflux at a temperature of 50° C., and reflux for 1.5 h, and filter to obtain 1.63 g of a solid product with a yield of 85.9% and a purity of 97.1%.
Embodiment 2
[0018] Add 5g (0.027mol) of 2-chloro-4-methoxy-3-hydroxybenzaldehyde to a 250ml three-necked flask, add 25ml of dichloromethane, stir until dissolved, and dropwise add 12.5g (0.050mol) of BBr at -30°C 3 , after the dropwise addition, the reaction solution was placed at room temperature and stirred for 4.6h. Under an ice-water bath environment (-25°C), 65ml of methanol was slowly added dropwise. After 3h, the dropwise addition was completed. %) to adjust the pH of the reaction solution to 2~3, heated to reflux at a temperature of 55°C, and refluxed for 2.5 hours, and filtered to obtain 4.16 g of a solid product with a yield of 89.4% and a purity of 95.2%.
Embodiment 3
[0020] Add 5g (0.027mol) of 2-chloro-4-methoxy-3-hydroxybenzaldehyde to a 250ml three-necked flask, add 25ml of dichloromethane, stir until dissolved, and dropwise add 13.53g (0.054mol) of BBr at -20°C 3 , after the dropwise addition was completed, the reaction solution was placed at room temperature and stirred for 5 hours, and 85ml of methanol was slowly added dropwise in an ice-water bath environment (-20°C). ) adjust the pH of the reaction solution to 2~3, heat to reflux at a temperature of 60° C., and reflux for 2 hours, and filter to obtain 4.06 g of a solid product with a yield of 87.3% and a purity of 93.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com