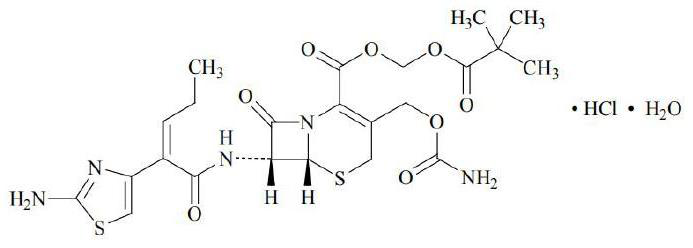
Cefcapene pivoxil granules and preparation method thereof
A technology of cefcapene axetil and granules, which is applied to pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. Problems such as poor stability, to achieve good business prospects, improved appearance, and high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
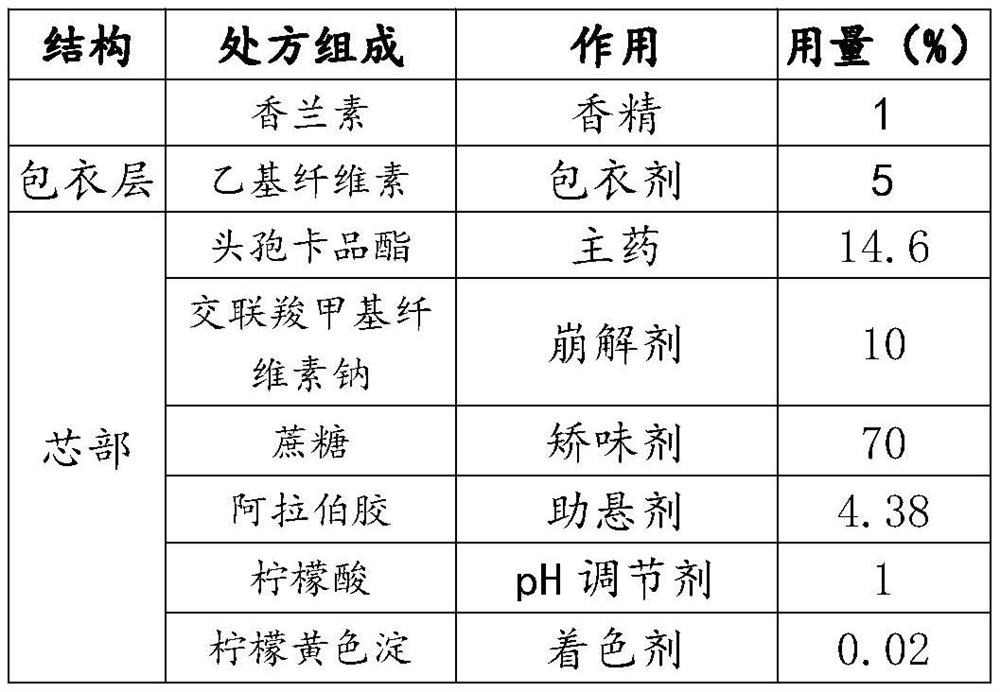
[0041] This embodiment provides a cefcapene pivoxil granule, which is composed of the components in weight percentage in Table 1.
[0042] The raw material proportioning of table 1 embodiment 1
[0043]
[0044] The consumption of essence in table 1 is that 1% is relative to the weight of cefcapene pivoxil granules, and the consumption 5% of coating agent is relative to the weight of granules containing medicine, and the consumption 14.6% of cefcapene pivoxil is relative to core weight. The weight ratio of cefcapene pivoxil to the cefcapene pivoxil granule is 13.77%, which is 10% when converted into cefcapene. The consumption notation of other embodiment and comparative example is the same.
[0045] The preparation method of the cefcapene pivoxil granule of the present embodiment may further comprise the steps:
[0046] (1) Cefcapene axetil is pulverized to a particle size of 176 μm in d90 and 83 μm in d50, and other materials are passed through a 60-mesh sieve.
[0047...
Embodiment 2
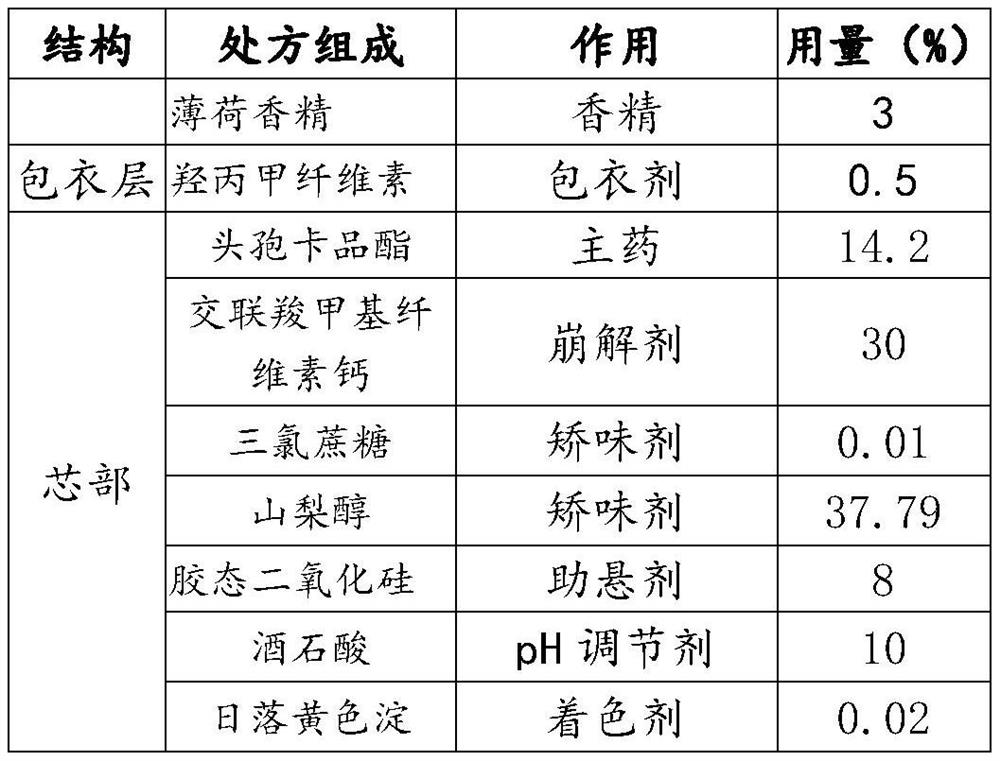
[0053] This embodiment provides a cefcapene pivoxil granule, which is composed of the components in weight percentage in Table 2.
[0054] The raw material ratio of table 2 embodiment 2
[0055]
[0056] The preparation method of the cefcapene pivoxil granule of the present embodiment may further comprise the steps:
[0057] (1) Cefcapene axetil is pulverized to a particle size of 174 μm in d90 and 66 μm in d50, and other materials are passed through a 60-mesh sieve.
[0058] (2) cefcapene axetil, croscarmellose calcium, sucralose, sorbitol, colloidal silicon dioxide, tartaric acid, sunset yellow lake are placed in the wet mixing granulator, stirred and mixed evenly, Add water under the conditions of stirring speed 200rpm and shearing speed 2000rpm for 15min to make soft material.
[0059] (3) Put the soft material in an extrusion spheronizer, and prepare granules under the conditions of a screen aperture of 0.7 mm, a roller speed of 5 rpm, a turntable speed of 800 rpm, a...
Embodiment 3
[0064] This embodiment provides a cefcapene pivoxil granule, which is composed of the components in weight percentage in Table 3.
[0065] The raw material ratio of table 3 embodiment 3
[0066]
[0067] The preparation method of the cefcapene pivoxil granule of the present embodiment may further comprise the steps:
[0068] (1) Cefcapene axetil is pulverized to a particle size of 153 μm in d90 and 52 μm in d50, and other materials are passed through a 60-mesh sieve.
[0069] (2) Cefcapene axetil, sodium carboxymethyl starch, lactose, xylitol, xanthan gum, lactic acid, and lemon yellow starch are placed in a wet mixing granulator, stirred and mixed evenly, at a stirring speed of 130rpm, shearing Add water under the condition of cutting speed 800rpm, stir and shear for 15min to make soft material.
[0070] (3) Put the soft material in an extrusion spheronizer, and prepare granules under the conditions of a sieve aperture of 0.4 mm, a roller speed of 20 rpm, a turntable spe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com