Preparation device and method of methyl 3-methyl-2-butenoate
A technology for the preparation of methyl dimethacrylate and equipment, which is applied in the field of preparation equipment for 3,3-methyl dimethacrylate, and can solve the problems of increasing the generation of by-products, increasing the amount of solid waste, increasing costs, and polluting the environment , to achieve a good yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
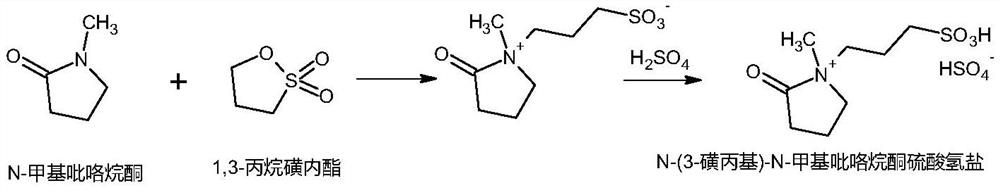
[0028] Preparation of N-(3-sulfopropyl)-N-methylpyrrolidone bisulfate ionic liquid:
[0029]
[0030] In a 500L reactor, put 72kg of N-methylpyrrolidone (0.726kmol) and 88.5kg of 1,3-propane sultone (0.725kmol), stir for 30min, then raise the temperature to 35°C for 24 hours. Cool down to below 10°C with ice-salt water, start to add 71.5kg of concentrated sulfuric acid (0.729kmol) dropwise, stir for 30 minutes after the dropwise addition, then raise the temperature to 80°C, keep the temperature for 14 hours, cool down to below 50°C after the reaction, add 232kg of methanol , after stirring evenly, put it in a bucket for subsequent use to obtain about 460kg of 50% N-(3-sulfopropyl)-N-methylpyrrolidone bisulfate ionic liquid methanol solution.
Embodiment 2~7
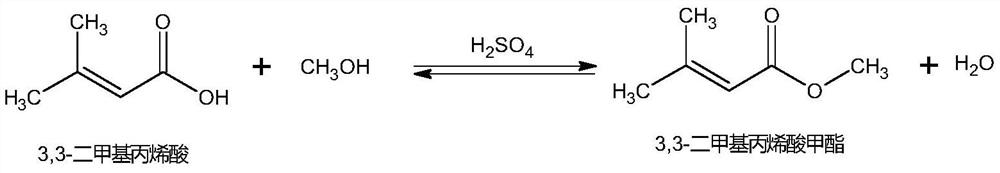
[0032] Preparation of 3,3-methyl dimethacrylate:
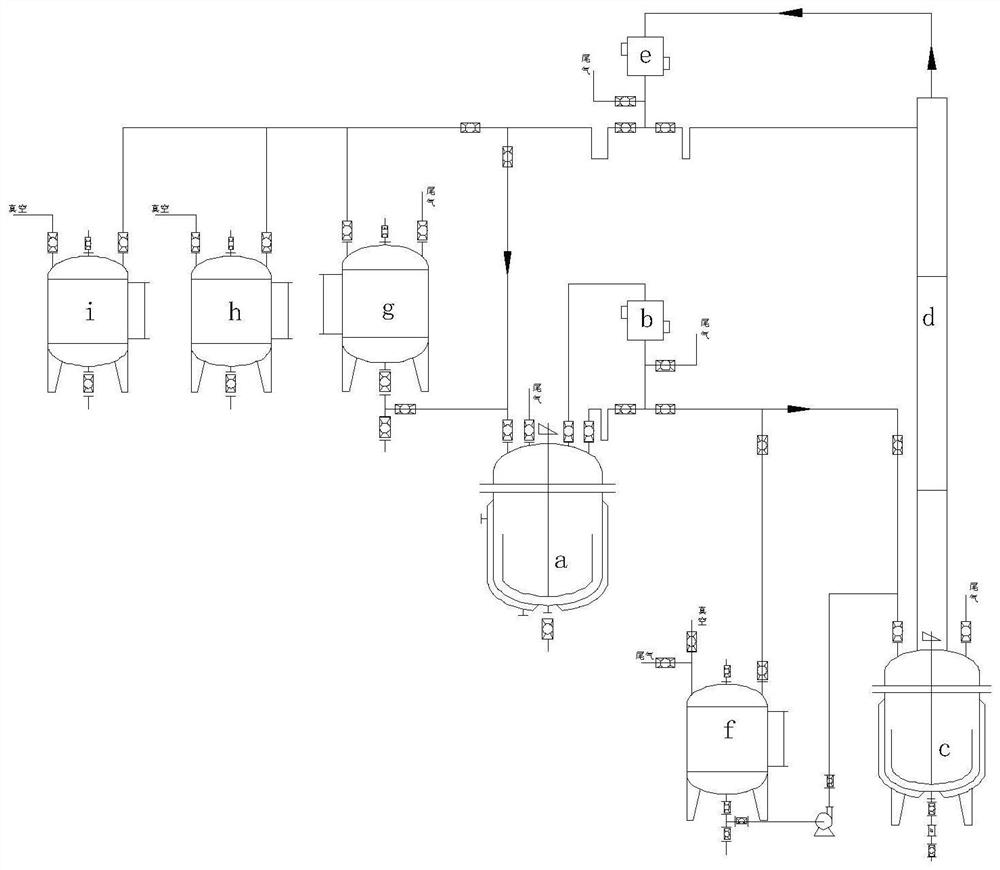
[0033] Step (1) In a 5000L reactor, 1000kg 3,3-dimethacrylic acid (9.99kmol), 1600kg methanol (49.94kmol) and 200kg 50% N-(3-sulfopropyl)-N-methyl Pyrrolidone bisulfate ionic liquid methanol solution, stir and heat up to 68-75°C, reflux reaction for 5 hours, change to distillation reaction, distill methanol, 3,3-dimethacrylate and water mixture to 3000L refined in the still.
[0034] Step (2) After the reaction kettle starts distillation reaction, the rectification kettle starts rectification, methanol is distilled from the top of the tower and flows into the reaction kettle, the temperature of the rectification kettle is controlled at 65-85°C, by controlling the temperature of the reaction kettle and adjusting The reflux ratio makes the methanol circulate in the system, and the 3,3-methyl dimethacrylate and water produced by the reaction accumulate in the rectification tank, and the reaction is circulated for 16 hours.
[0...
Embodiment 8
[0040] Preparation of 3,3-methyl dimethacrylate:
[0041] Step (1) In a 1000ml reaction flask, put 200g of 3,3-dimethacrylic acid (2mol), 300g of methanol (9.36mol) and 20g of N-(3-sulfopropyl)-N-methylpyrrolidone hydrogensulfate in sequence Salt ionic liquid, heat up to 75°C, reflux reaction for 6 hours, then change to distillation reaction, distill the mixture of methanol, 3,3-methyl methacrylate and water into the rectification bottle while reacting.
[0042] Step (2) After starting the distillation reaction, the rectifying bottle starts to rectify, and methanol is distilled from the top of the tower and flows back into the reaction bottle. The internal temperature of the rectifying bottle is controlled at 65 to 85°C. Methanol is circulated in the system, and the 3,3-methyl methacrylate and water produced by the reaction are gradually accumulated in the rectifying bottle, and the reaction is circulated for 12 hours.
[0043] Step (3) After the reaction is completed, the re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com