Compound Olerafuran A in herba portulacae as well as extraction and separation method and application of compound Olerafuran A
A separation method and compound technology, applied in the direction of food ingredients containing natural extracts, applications, drug combinations, etc., can solve the problem of low structural novelty, and achieve the effect of environmentally friendly process methods, simple and fast operation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Extracting and separating compound Olerafuran A from purslane and its extraction and separation method
[0034] A compound Olerafuran A extracted and isolated from purslane, the molecular formula is C 18 h 30 o 5 , named Olerafuran A, the chemical structure is:
[0035] Described new compound is called OlerafuranA according to structure, and table 1 is the NMR data of this new compound: 1 H-NMR with 13 C-NMR in DMSO.
[0036] Table 1. NMR data of the new compounds of the present invention.
[0037]
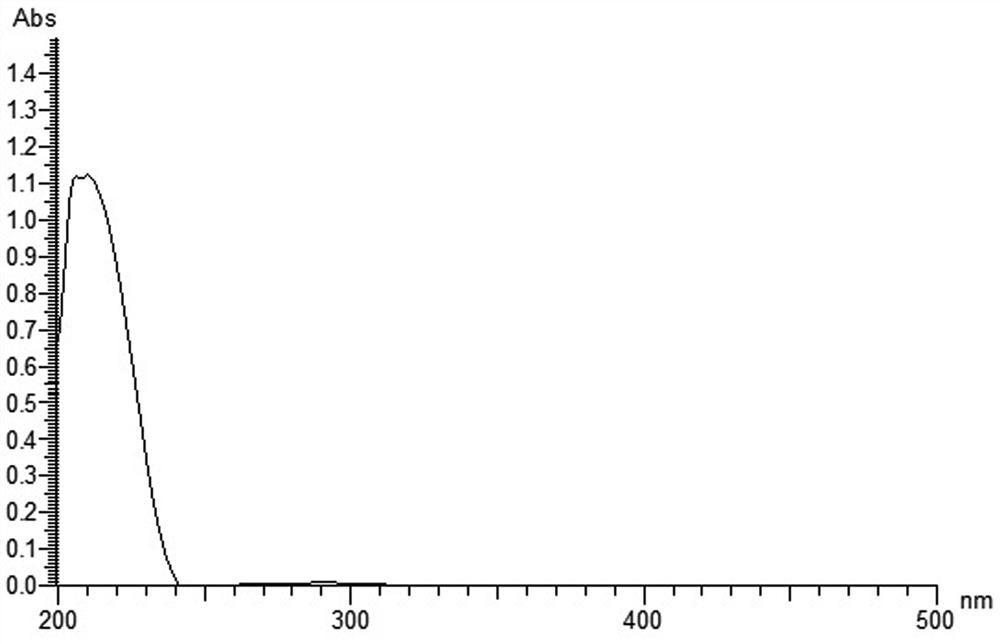
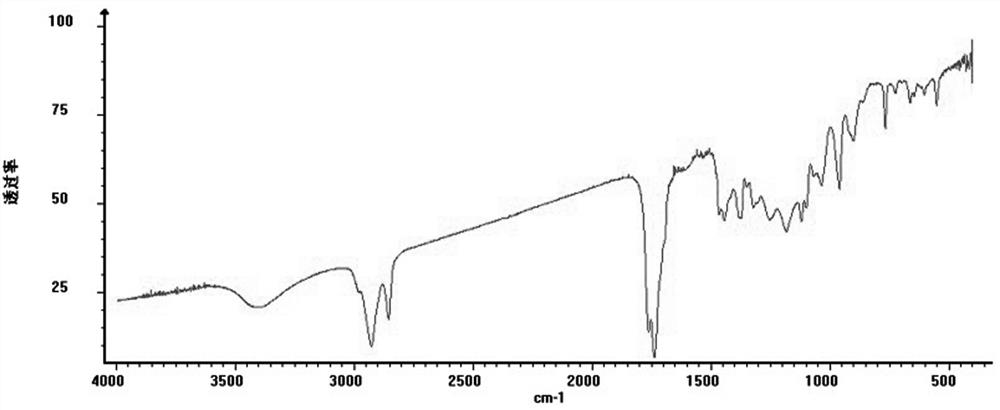
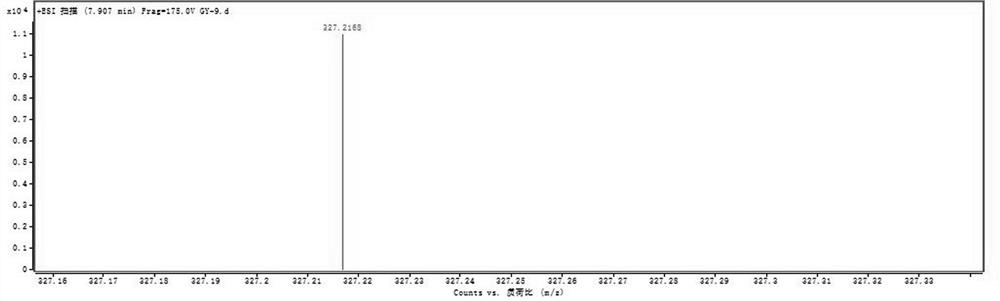
[0038] Olerafuran A: yellow oil, easily soluble in methanol, slightly soluble in chloroform. UV(MeOH)λ max :213nm. IR(KBr)v max 3404, 2983, 2928, 2854, 1763, 1736, 1468, 1443, 1378, 1252, 1178, 768cm -1 . HR-ESI(+)-TOF-MS gave m / z: 327.2168, [M+H] + The quasi-molecular ion peak has a molecular weight of 327.2167. to combine 1 H-NMR, 13 According to C-NMR and DEPT data, it is speculated that the possible molecular formula of the compound is C ...
Embodiment 2
[0047] Example 2 Anticholinesterase effect of compounds of the present invention.
[0048] 1. Main materials.
[0049] 1.1. Drugs and reagents.
[0050] The compound Olerafuran A used in the experiment was prepared by the above method with a purity of 90-99%. Sodium dihydrogen phosphate, disodium hydrogen phosphate (Sinopharm Chemical Reagent Co., Ltd.), physostigmine (Hanxiang Biotechnology), phosphorus-5,5 '-Dithiobisnitrobenzoic acid (Dithiobisnitrobenzoic acid, DTNB, Shanghai Jinsui Biotechnology Co., Ltd.), acetylcholinesterase (AChE) and iodide thioacetylcholine (Acetylthiocholine iodide, ATCI, Dalian Meilun Biotechnology Co., Ltd).
[0051] 1.2 Grouping.
[0052] Divided into negative control group, positive control group and experimental group, each group.
[0053] 2 Experimental methods.
[0054] 2.1 Sample preparation.
[0055] Precisely weigh 1 mg of samples Olerafuran A and physostigmine, respectively, and use methanol as a solvent to prepare five gradient co...
Embodiment 3
[0062] Example 3 Anti-tumor effect of the new compound of the present invention.
[0063] 1 main material.
[0064] 1.1 Drugs and reagents.
[0065] The new compound Olerafuran A used in the experiment was prepared by the above-mentioned method with a purity of 90-99%. It was accurately weighed and diluted with DMSO to the solution required for each dosage group below. DMEM high-glucose medium, fetal bovine serum (Hyclone Company of the United States); penicillin and streptomycin (Hangzhou Sijiqing Company).
[0066] 1.2 Cell lines.
[0067] Human colon cancer cell Caco-2, human breast cancer cell MCF-7, human gastric cancer cell BGC-823, human lung adenocarcinoma cell SPC-A1, human liver cancer cell BEL-7402, human cervical cancer cell Hela-229, ovarian cancer cell Ho-8910, human oral epidermoid carcinoma cells KB (Shanghai Cell Bank, Chinese Academy of Sciences).
[0068] 1.3 Grouping.
[0069] Divided into control group, experimental group and zero adjustment group (cu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of unsaturation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com