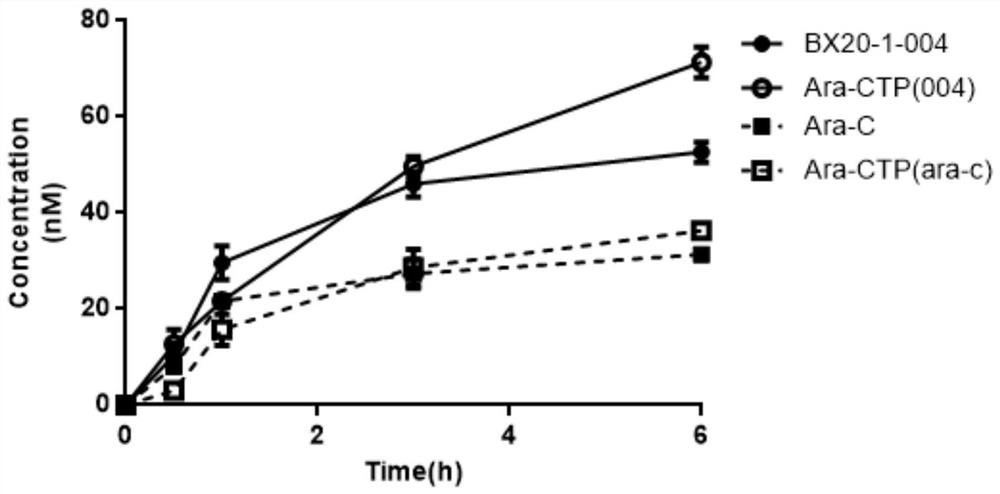
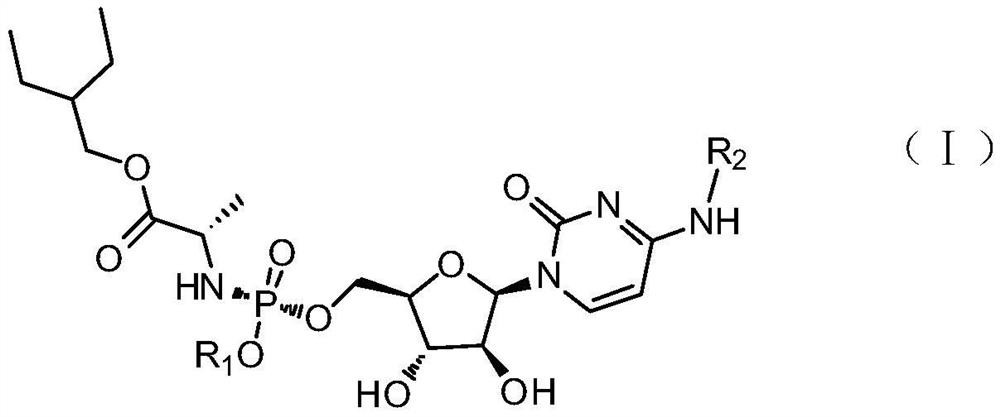
Cytarabine analogue and preparation method and application thereof
A cytarabine, a technology similar in structure, applied in the field of cytarabine structural analogs and their preparation, can solve the problems of limiting the clinical use of cytarabine, not significantly improving the survival rate, organ toxicity and side effects, etc. Effective blood drug concentration, preparation method is simple and easy to operate, high bioavailability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
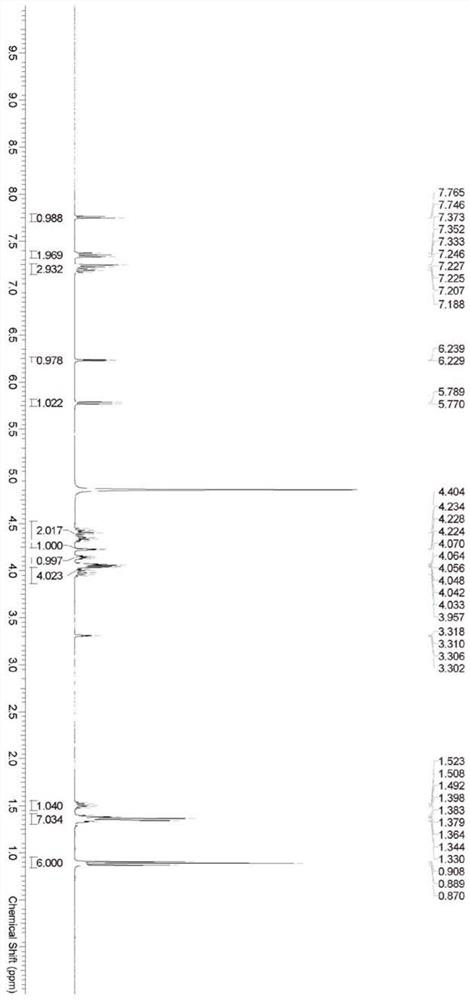
[0072] Example 1 Synthesis of Cytarabine Structural Analogue P1
[0073] The synthetic route is shown in the following formula:
[0074]
[0075] Method: Cytarabine (compound 1, 5.0g, 20.56mmol) and compound 2 (10.72g, 30.84mmol, 1.5eq.) were dissolved in anhydrous THF (tetrahydrofuran, 50mL), N 2 The temperature was lowered to -78°C under protection, and 1-methylimidazole (4.39g, 53.46mmol, 2.6eq.) was slowly added dropwise, then stirred at -78°C for 2 hours and then naturally warmed to room temperature (20°C) for 12 hours. The reaction solution was concentrated under reduced pressure at 40°C, diluted with DCM (dichloromethane), washed successively with dilute hydrochloric acid (0.5mol / L), water, and saturated sodium chloride solution, the organic phases were combined, dried over anhydrous sodium sulfate, and reduced to 40°C. After concentration under reduced pressure, it was purified by column chromatography (DCM / MeOH=20 / 1-10 / 1, V / V, MeOH is methanol) to obtain a white p...
Embodiment 2
[0076] Example 2 Synthesis of Cytarabine Structural Analogue P2
[0077] The synthetic route is shown in the following formula:
[0078]
[0079] Method: Compound P1 (3.0 g, 5.41 mmol) and compound 3 (2.32 g, 6.49 mmol, 1.2 eq.) were dissolved in anhydrous DCM (tetrahydrofuran, 50 mL), N 2 The temperature was lowered to 0°C under protection, and 1-propylphosphoric anhydride (50% ethyl acetate solution) (6.88g, 1-propylphosphoric anhydride T 3 P 10.82mmol, 2.0eq.), then naturally warmed to room temperature (20°C) for 1 hour reaction. The reaction solution was saturated NaHCO 3 The solution was quenched, the aqueous phase was extracted with DCM (dichloromethane, 50mL×2), the organic phases were combined, dried over anhydrous sodium sulfate, concentrated under reduced pressure at 40°C, and column chromatography (PE / EA=20 / 1~1 / 1, PE is petroleum ether, EA is ethyl acetate) and purified to obtain intermediate P1a. Dissolve the intermediate P1a in methanol (50 mL), add Pd / C (...
Embodiment 3
[0080] Example 3 Synthesis of Cytarabine Structural Analogue P3
[0081] The synthetic route is shown in the following formula:
[0082]
[0083] Method: Cytarabine (compound 1, 5.0g, 20.56mmol) and compound 4 (16.16g, 30.84mmol, 1.5eq.) were dissolved in anhydrous THF (tetrahydrofuran, 50mL), N 2 The temperature was lowered to -78°C under protection, and 1-methylimidazole (4.39g, 53.46mmol, 2.6eq.) was slowly added dropwise, then stirred at -78°C for 2 hours and then naturally warmed to room temperature (20°C) for 12 hours. The reaction solution was concentrated under reduced pressure at 40°C, diluted with DCM (dichloromethane), washed successively with dilute hydrochloric acid (0.5mol / L), water, and saturated sodium chloride solution, the organic phases were combined, dried over anhydrous sodium sulfate, and reduced to 40°C. After concentrated under reduced pressure, it was purified by column chromatography (DCM / MeOH=20 / 1-10 / 1, V / V) to obtain a white powdery compound. L...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com