LC-MS/MS (Liquid Chromatography-Mass Spectrometry/Mass Spectrometry) determination method of aloesin in rat plasma
A kind of aloe verain and determination method technology, applied in the field of aloe verain, can solve the problems of backward analysis technology, less aloe verain, no pharmacokinetic parameters provided, etc., and achieve the effect of accurate detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
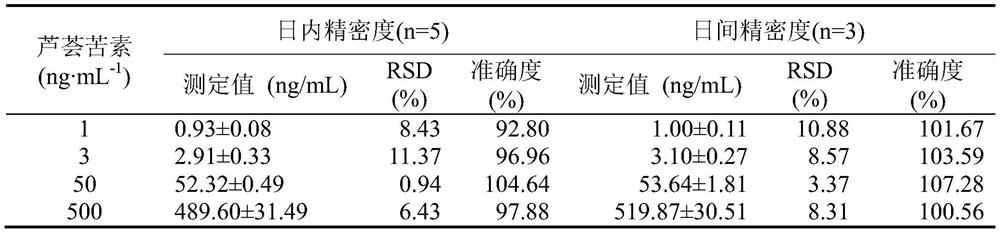
[0032] 1 Instruments, reagents and experimental animals
[0033] 1.1 Instrument
[0034] Nexera XR UHPLC liquid chromatograph (Shimadzu Corporation, Japan) is connected in series with AB-SCIEX API 4000+ quadrupole mass spectrometer through Turbo V ion source interface, and Analyst software controls data acquisition and processing (AB Corporation, USA). 5922 type refrigerated centrifuge (KUBOTA, Japan), XS105DU 1 / 100,000 electronic analytical balance (Mettler-Toledo, Switzerland), LabTower EDI15 ultra-pure water machine (Thermo, USA), Vibrax circular oscillator (Aika, Germany ). AMS small animal anesthesia machine (Beijing Gene & I).
[0035] 1.2 Drugs and experimental animals
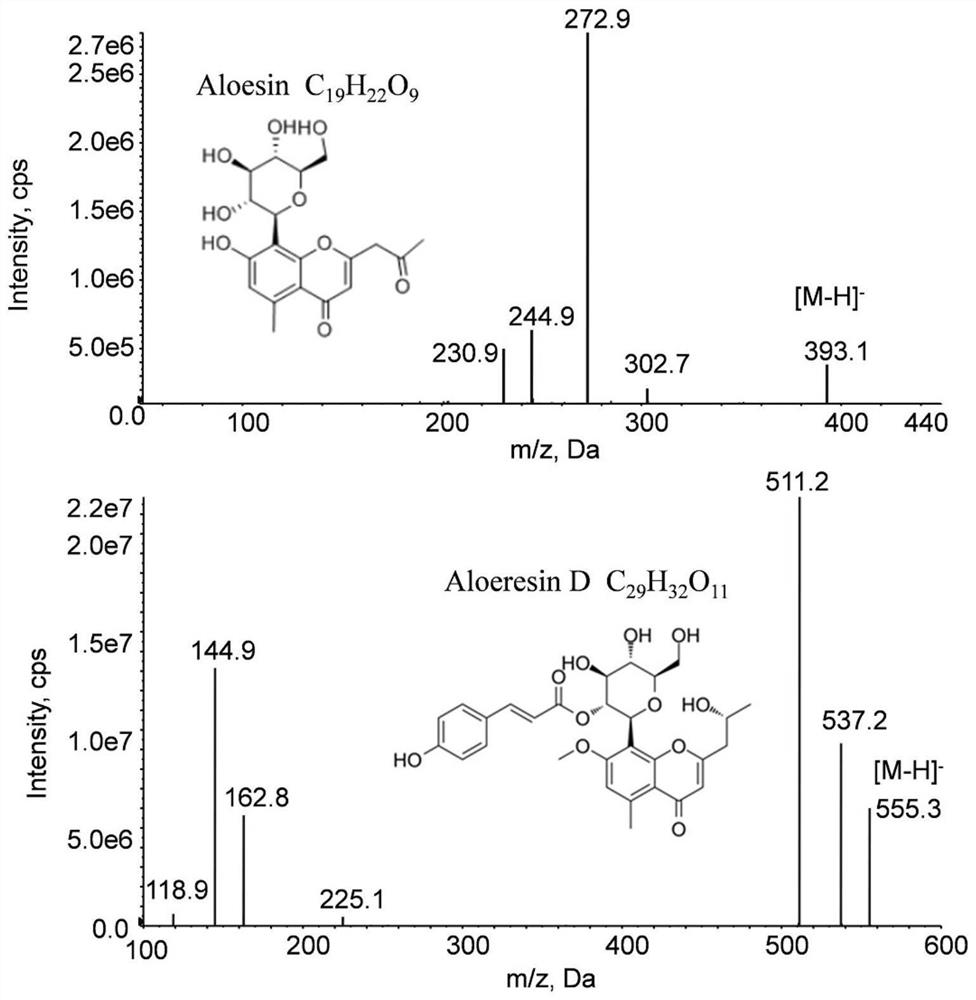
[0036] The aloesin reference substance was provided by Hao Rui Chemical (Shanghai) Co., Ltd.; the aloeresin D (Aloeresin D) reference substance was provided by Chengdu Pufeide Biotechnology Co., Ltd., batch number: 19072605. The purity of the reference substances were all ≥98%. Chromatographic grad...
Embodiment 2
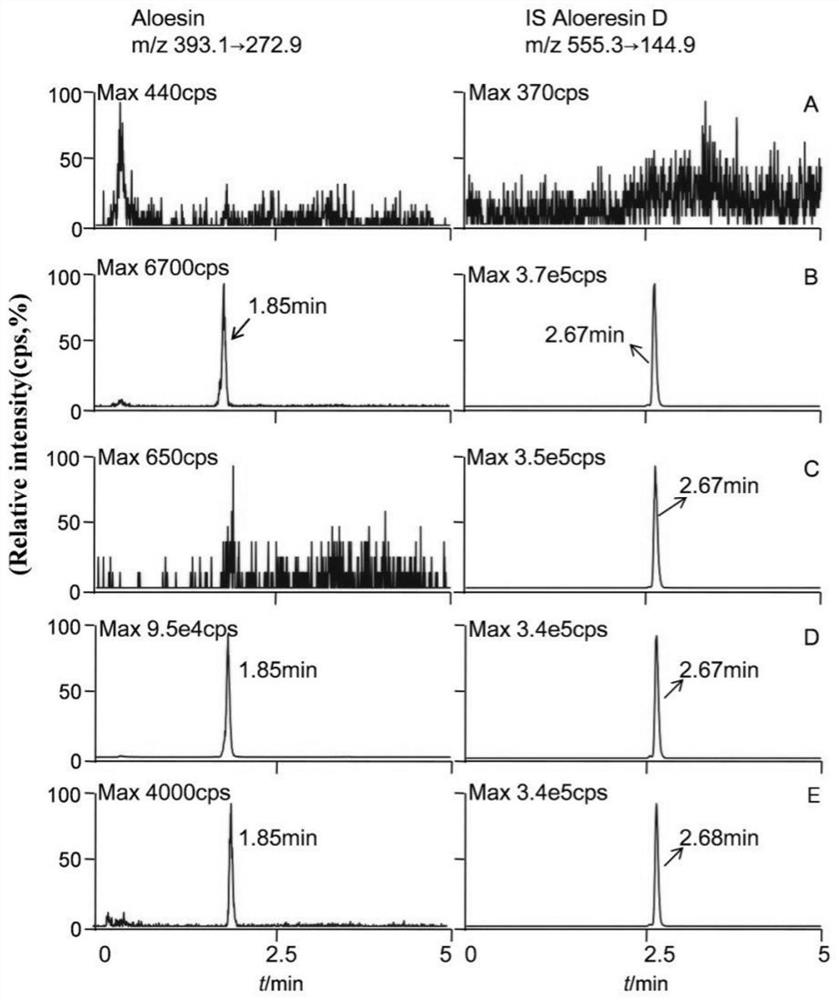
[0082] The difference between this example and Example 1 is that mobile phase A is 1.0‰ (v / v) formic acid aqueous solution. After testing, the results showed that the peak shape of aloin and aloin D was good without mutual interference, and the endogenous substances in plasma had no obvious influence on the signals of aloin and aloin D.
Embodiment 3
[0084] The difference between this example and Example 1 is that the concentration of the methanol solution containing the internal standard aloin D is 100 ng / mL. After testing, the results showed that the peak height and peak area of aloin D increased by about 1 times, which can be used for the effective quantification of aloesin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com