External preparation of lidocaine-prilocaine medicinal composition and application thereof
A technology for external preparations and prilocaine, which is applied in the direction of drug combinations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of cumbersome operation and clothing erasure, and achieve good safety, Reduce the incidence rate and reduce the effect of toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
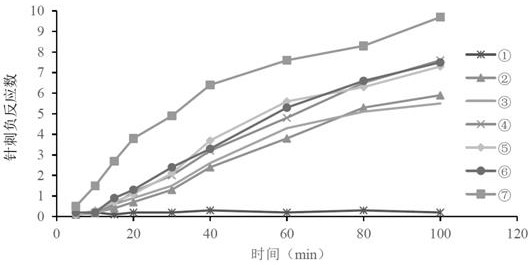
Image
Examples
Embodiment 1
[0058] Embodiment 1: lidocaine-prilocaine patch (solvent base)
[0059] Prescription composition:
[0060]
[0061] Preparation process: Take lidocaine and prilocaine in the prescribed amount into a container, heat to 40-50°C, stir and mix until they are completely melted, forming a transparent oily eutectic liquid. Take the prescribed amount of DURO-TAK 180-129A, kaolin, tocopherol, propylene glycol, methyl p-hydroxybenzoate and lidocaine-prilocaine oily eutectic into a mixing container, stir and mix evenly to obtain a drug-containing layer. The drug-containing layer is evenly coated on the polyethylene terephthalate PET film, laminated with non-woven fabric, and cut into the required size to obtain the lidocaine-prilocaine patch.
Embodiment 2
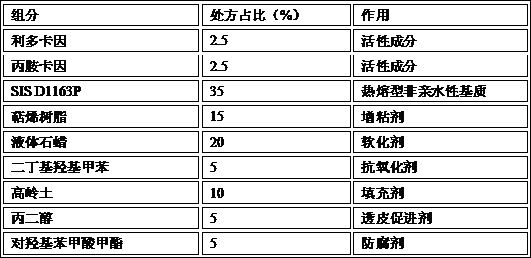
[0062] Embodiment 2: lidocaine-prilocaine patch (hot-melt matrix)
[0063] Prescription composition:
[0064]
[0065] Preparation process: Take lidocaine and prilocaine in the prescribed amount into a container, heat to 40-50°C, stir and mix until they are completely melted, forming a transparent oily eutectic liquid. Take the prescribed amount of SIS D1163P, terpene resin, liquid paraffin, dibutyl hydroxytoluene, kaolin, propylene glycol and methyl p-hydroxybenzoate, heat to 150°C to melt and mix well. Subsequently, the lidocaine-prilocaine oily co-melt is added into a mixing container, stirred and mixed evenly to obtain a drug-containing layer. The drug-containing layer is evenly coated on the polyethylene terephthalate PET film, laminated with non-woven fabric, and cut into the required size to obtain the lidocaine-prilocaine patch.
Embodiment 3
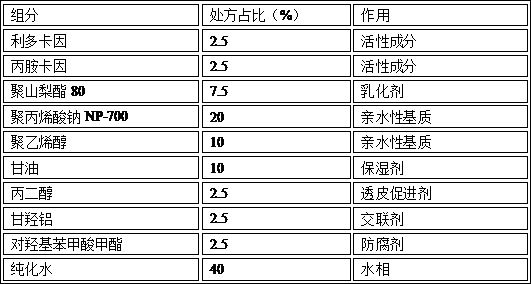
[0066] Embodiment 3: lidocaine-prilocaine gel plaster
[0067] Prescription composition:
[0068]
[0069] Preparation process: Take lidocaine and prilocaine in the prescribed amount into a container, heat to 40-50°C, stir and mix until they are completely melted, forming a transparent oily eutectic liquid. Put polysorbate 80, lidocaine-prilocaine co-melt and appropriate amount of purified water in a mixing container, stir homogeneously to emulsify, and prepare an O / W emulsion.
[0070] Dissolve the prescribed amount of sodium polyacrylate NP-700 and polyvinyl alcohol in purified water, fully mix aluminum glycerol, propylene glycol, kaolin, glycerin, and methyl p-hydroxybenzoate, and then add it to the above purified aqueous solution to cross-link to form a gel. Gel base. Then add the O / W type emulsion into the gel matrix, stir and mix evenly to obtain the drug-containing layer. The drug-containing layer is uniformly coated on a polyethylene terephthalate PET film, and l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com