Helicobacter pylori multi-epitope series fusion protein LHUC as well as preparation method and application thereof
A technology of Helicobacter pylori and fusion protein, applied in the field of biopharmaceuticals, can solve problems such as difficulty in preparation, achieve strong immune effect, enhance immunogenicity, and avoid toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
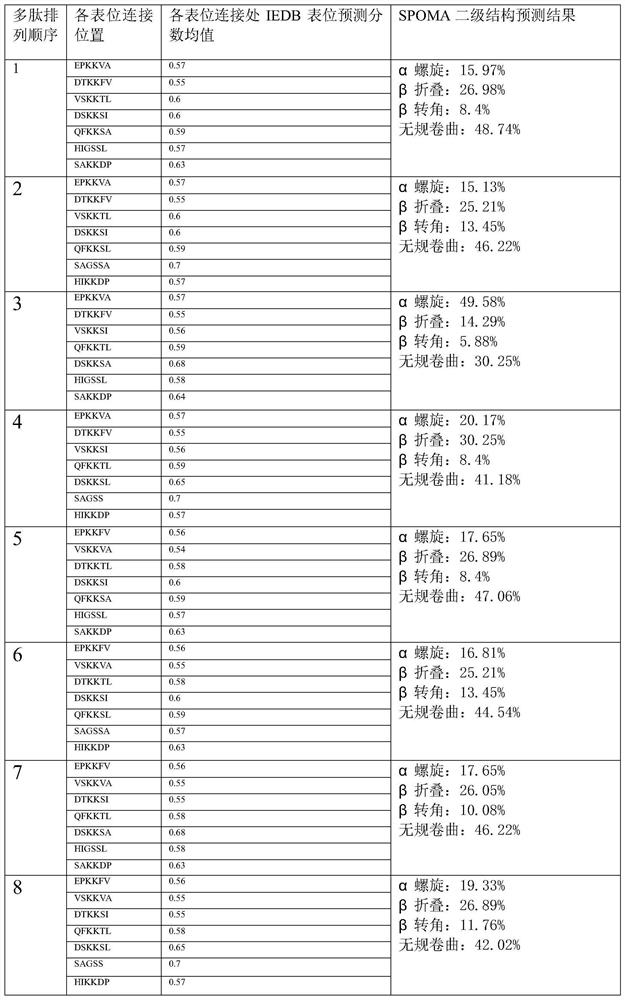
[0044] According to the immune protection mechanism of Helicobacter pylori infection, three T cell epitopes (HpaA 154-171 , UreB 237-251 , UreB 546-561 ) and five B cell epitopes (UreB 349-363 , UreB 327-334 、CAT 394-405 、CAT 387-397 and HpaA 132-141 ). By analyzing the antigenicity between each epitope and adding amino acids KK and GS to avoid the interaction between each epitope, optimize the sequence of each epitope ( Figure 1-Figure 2 ), obtain the sequence 1 polypeptide shown in SEQ ID NO:3 as an alternative peptide chain, named as HUC. Finally, the mucosal adjuvant LTB was connected to the N-terminus of the multi-epitope peptide HUC, separated by a flexible peptide PQDPPP in the middle. The isoelectric point, antigenicity, hydrophilicity, stability and advanced structure of the fusion peptide were analyzed by bioinformatics tools such as DNASTAR, Expasy and MOE. According to the predicted results of physical and chemical properties, the molecular mass is 27.8KD...
Embodiment 2
[0045] Example 2 Recombinant plasmid construction
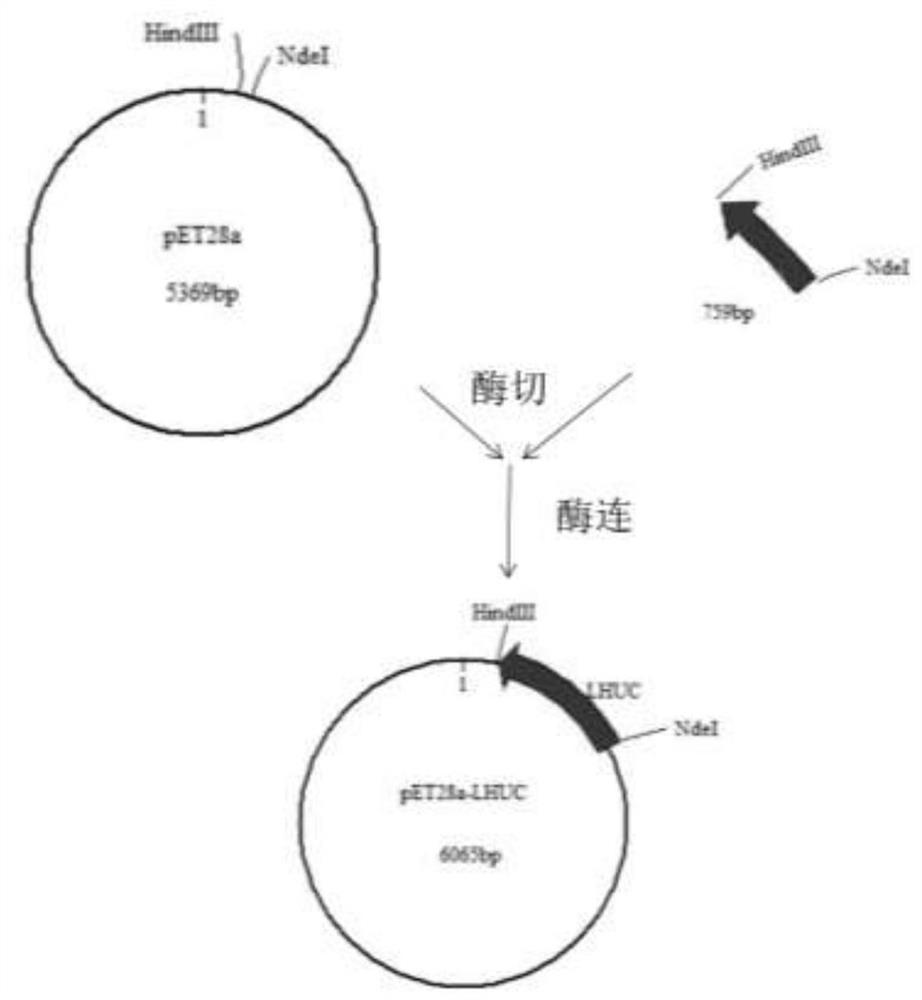
[0046] According to the codon preference of Escherichia coli, the nucleotide sequence corresponding to the amino acid sequence of the vaccine protein was codon-optimized and synthesized by Qingke Biotechnology Co., Ltd. A NdeI restriction site was introduced at the 5' end of the sequence, a HindIII restriction site was introduced at the 3' end, and the sequence was inserted into the pET28a expression vector. The constructed plasmid was transformed into Escherichia coli DH5ɑ, cultured overnight at 37°C on a Kana-resistant plate, and a single clone was picked for colony PCR and plasmid double-enzyme digestion verification. Plasmids were digested and sequenced for verification. Enzyme digestion electropherogram reference Figure 4 .
Embodiment 3
[0047] Example 3 Preparation and Purification of Multiple Tandem Epitope Vaccines
[0048] (1) Verification of multi-tandem epitope vaccine expression
[0049] The recombinant plasmid constructed in Example 2 was transformed into Escherichia coli BL21(DE3), a single clone was picked and inoculated in LB medium containing 50 ug / ml kanamycin, and shaken overnight at 37°C. Inoculate in LB medium containing 50ug / ml kanamycin at a ratio of 1:100, culture with shaking at 37°C, add IPTG to a final concentration of 1mM when the OD 600 value is between 0.6-0.8, and induce 1 and 2 respectively , 3, 4, 5 hours later collect bacteria. Bacteria and protein loading buffer were mixed, boiled in boiling water for 10 minutes and centrifuged, the supernatant was collected and tested by 12% SDS-PAGE, the results were as follows Figure 5 shown.
[0050] (2) Expression and purification of multi-tandem epitope vaccine
[0051] Cultivate 1L of bacteria according to the above method, and after i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com