Preparation method of 3-hydroxymethyl cefotaxime
A technology of hydroxymethyl cefotaxime and cefotaxime, which is applied in the directions of resisting vector-borne diseases, organic chemistry, fermentation, etc., can solve the problems of many steps, complicated operations, etc., and achieves mild reaction conditions, simple and easy-to-control operations, The effect of high product conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
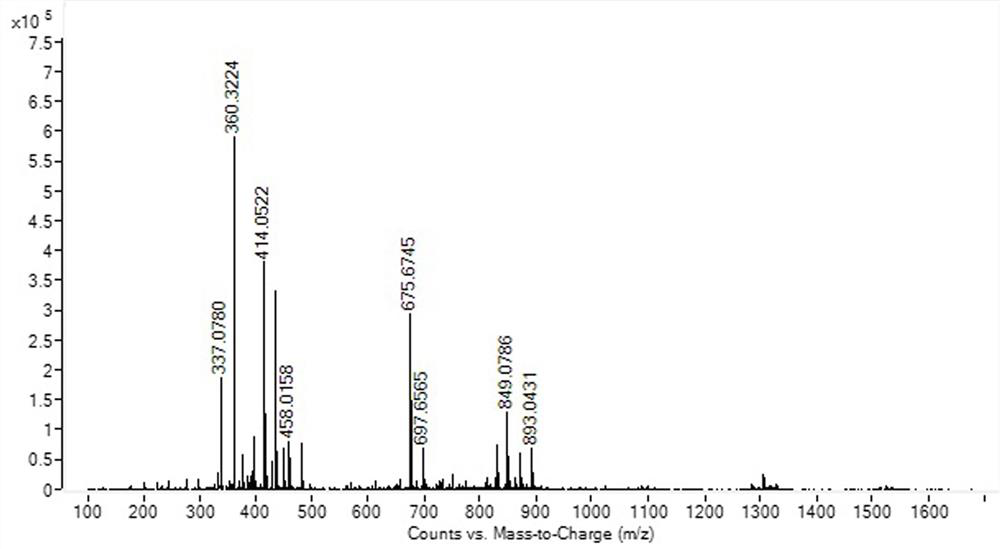
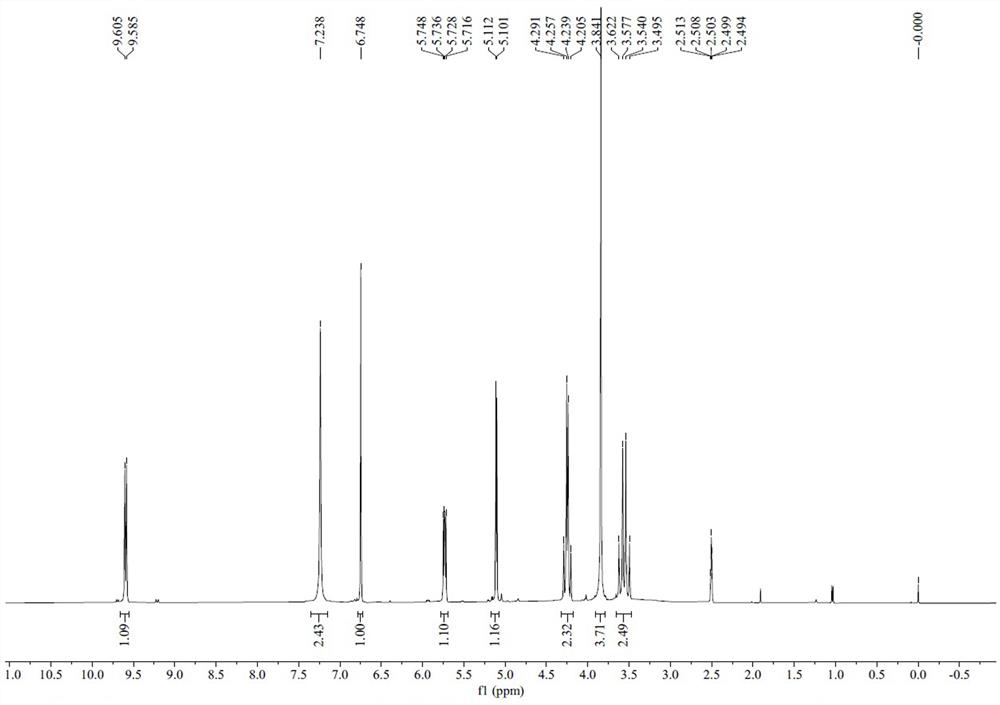
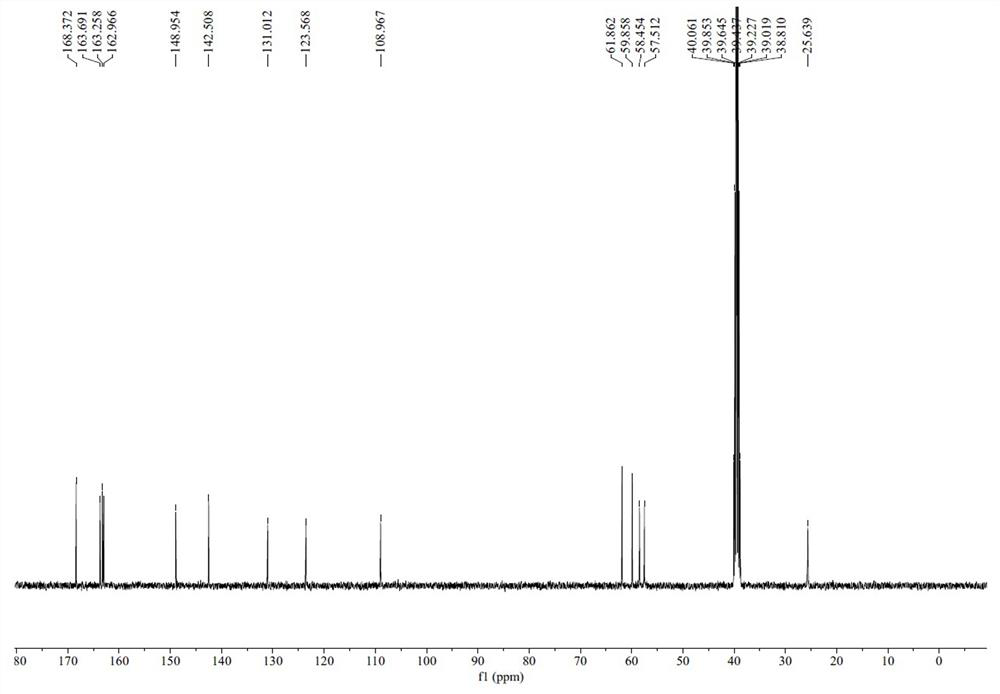
Image
Examples
Embodiment 1
[0024] Example 1: In a 500 ml of four-mouth flasks, 20 g of cefosporoalic acid and 160 ml of purified water were added, and the temperature of 25 ° C to 30 ° C was added, stirred well, and the 3 mol / L ammonia solution was added to 7.0 to 7.5, stirred after 5 min. 4 g of ceftriacin C-denyloy was added, and the pH was allowed to be finely adjusted with 3 mol / L ammonia solution, and the reaction was completed when the pH was maintained for 5 minutes, then filtered, filtered, filtered, and filtered to the cefosporin C to acerase. Collect the filtrate;
[0025] When the above filtrate temperature was 10 to 15 ° C, a mixed solution of 5 ml of 36% concentrated hydrochloric acid and 7.5 ml of isopropyl alcohol was added dropwise, and the pH was adjusted to 2.5, crystallization, crystalline 45min filtration, and then purified with 30 ml of purification water After drying temperature 55 ° C, dried under -0.090 MPa, 5 to 10 hours, to moisture less than 2.0%, resulting in pale yellow powd...
Embodiment 2
[0030] Example 2: In a 500 ml of four-mouth flasks, 20 g of cefradoxamic acid and 200 ml of purified water were added, and the temperature of 25 ° C to 30 ° C was added, and the mixture was mixed well, and the aqueous solution of 3 mol / L ammonia was added to 7.0 to 7.5, stirred for 5 min. After 6 g of ceftriaciacin C to acerase, the pH was continued with 3 mol / L ammonia solution to maintain at 7.0 to 7.5, and the reaction was completed when the pH was maintained for 5 minutes, then filtered, filtered, filtered to the cefosporin C to acerase , Collect filtrate;
[0031]When the above filtrate temperature was 10 to 15 ° C, a mixed solution of 6 ml of 36% concentrated hydrochloric acid and 6 ml of ethanol was added dropwise, and the pH was adjusted to crystalline, crystalline 45min was filtered, and then purified with 20 ml of purification, after washing, The drying temperature was 50 ° C, and the vacuum dried under -0.095 MPa was dried for 5 to 10 hours, and the water was less t...
Embodiment 3
[0032] Example 3: In a 500 ml of four-mouth flasks, 20 g of cefide sodium cefotaxime sodium celaroxime sodium and 240 ml of purified water were added, and the temperature of 25 ° C ~ 30 ° C was controlled, and the mixture was mixed well, and the aqueous solution of 3 mol / L ammonia was added to 7.0 to 7.5, stirred for 5 min. After the addition of 8 g of ceftriaridin C-deacetylase, the pH was continued with 3 mol / L ammonia solution, and the reaction was maintained at 7.0 to 7.5, and the reaction was completed when the pH was maintained for 5 minutes, then filtered, filtered, and filtered to the cefosporin C to aceralase. , Collect filtrate;
[0033] When the above filtrate temperature was 10 to 15 ° C, 4 ml of 85% phosphoric acid and a mixed solution composition of 8 ml of methanol were added dropwise, and the pH was adjusted to crystalline, crystalline 45min, filtered, then purified with 25 ml, after drying The temperature was 60 ° C, and the vacuum dried under -0.085MPa was dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com