Anti-cd123 Nanobody and Its Application
A nanobody and chimeric antigen receptor technology, applied in the field of biomedicine, can solve problems such as failure to achieve ideals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1 Construction, panning and ELISA preliminary screening of phage nanobody library
[0073] (1) Construction of phage nanobody library
[0074] In this example, the extracellular region of CD123 antigen was used to immunize Bactrian camels, and after ELISA was used to verify the titer, 200 mL of peripheral blood was extracted; lymphocytes were sorted from peripheral blood to obtain peripheral blood mononuclear lymphocyte precipitation, and RNA extraction was performed;
[0075] Using the extracted RNA as a template, The first-strand cDNA was synthesized by reverse transcriptase, and then the VHH gene was amplified by nested PCR; the amplified VHH gene was inserted into the pMECS phage display vector, and TG1 competent cells were electrotransformed. Cultures were evenly spread on LB / AMPGLU plates;
[0076] After the bacteria grow out, collect the bacterial moss, add 1 / 3 volume of 50% glycerol, mix and distribute, and store at -80 °C, that is, the storage capacity...
Embodiment 2
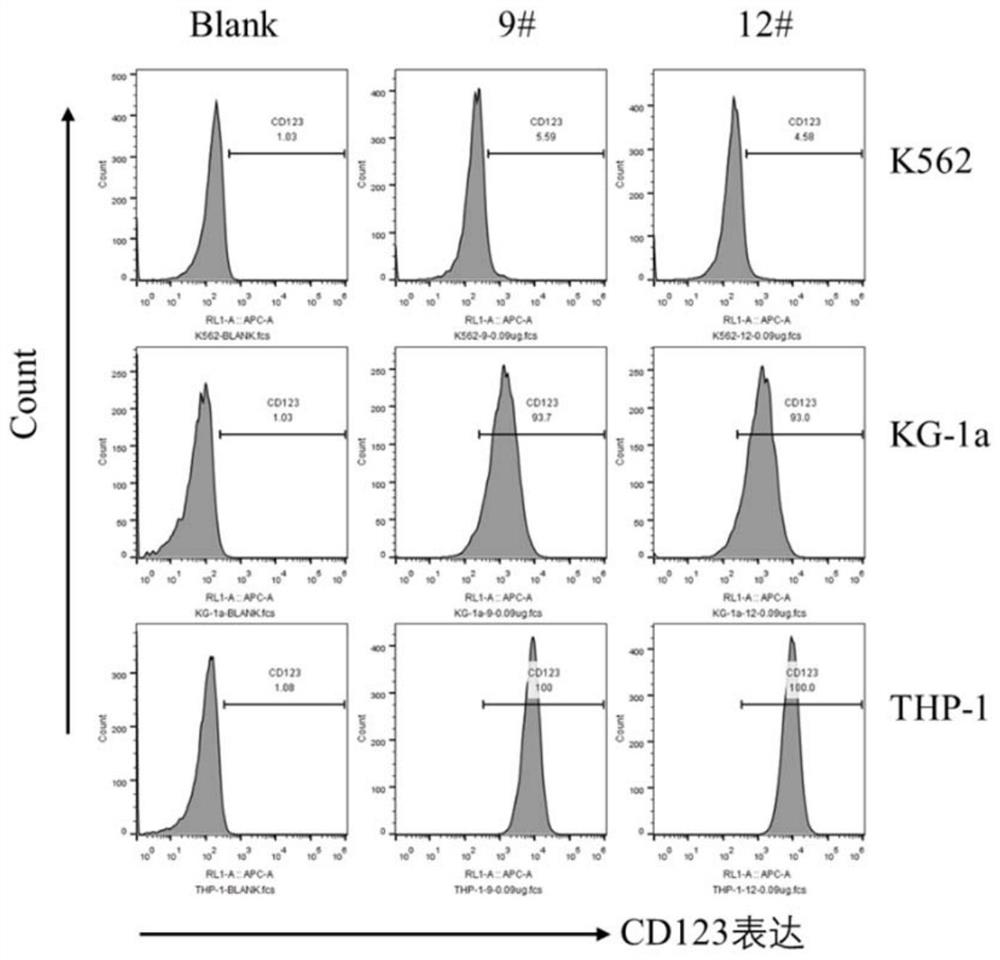
[0089] Example 2 FACS screening of candidate clones
[0090] In this example, the cells were cultured according to the standard cell culture protocol:
[0091] Use trypsin to digest the cells to prepare a CD123-positive or CD123-negative cell suspension, centrifuge at 300g for 5 min to remove the culture medium, and resuspend the cells in Flow Buffer to a cell concentration of 2×10 6 pcs / mL;
[0092] Add 2 x 10 to each well of a V-bottom 96-well plate 5 Cells were centrifuged at 300 g for 5 min to remove the supernatant, and the crude VHH antibody extract was added to resuspend the cells, and incubated at 4°C for 1 h;
[0093] Centrifuge at 300 g for 5 min to remove the supernatant, resuspend the cells in Flow Buffer, add 100 μL of APC anti-his antibody (2 μg / mL) diluted in Flow Buffer, and incubate at 4°C for 1 h;
[0094] After washing the cells three times with Flow Buffer, resuspend the cells in 200 μL of Flow Buffer for flow cytometry.
Embodiment 3
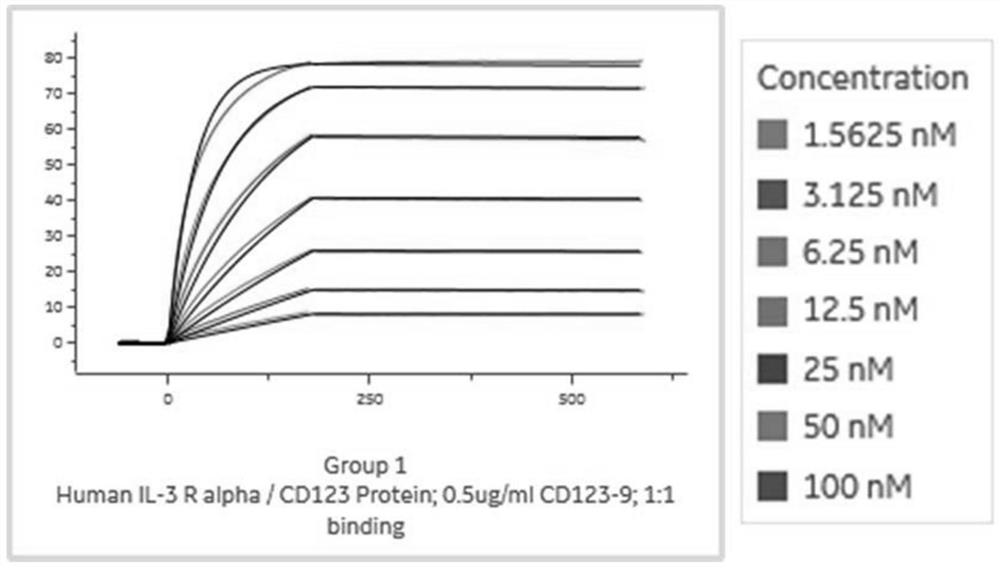
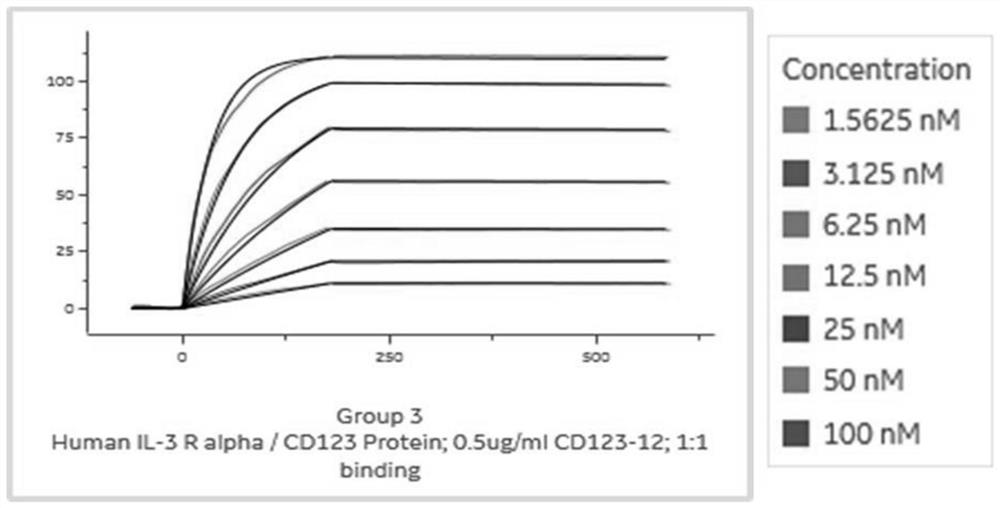
[0095] Example 3 Expression, purification and affinity determination of VHH-mIgG2a Fc Nanobody
[0096] In order to further identify the screened antibodies, this example constructs a VHH-expressing vector C-4pCP.Stuffer-mCg2a-FC (with a mouse Fc tag), and the steps are as follows:
[0097] PCR amplification of the anti-CD123 heavy chain variable region encoding gene, wherein the upstream primer of CD123-9 (SEQ ID NO: 6) is HD-F, the downstream primer is HD-B12-R2, CD123-12 (SEQ ID NO: 6) 7) The upstream primer is HD-F, and the downstream primer is HD-CD-12-R. The sequence is shown in Table 2. The PCR reaction system is shown in Table 3. The reaction conditions are pre-denaturation at 95°C for 1min, and denaturation at 95°C for 10s. , annealing at 55°C for 10s, extension at 72°C for 10s, 30 cycles, extension at 72°C for 5 min, and storage at 4°C.
[0098] Table 2
[0099]
[0100] table 3
[0101]
[0102] The empty vector was digested with enzyme at 37°C for 6 hours....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com