Oxaliplatin conjugate as well as preparation method and application thereof
An oxaliplatin and conjugate technology, applied in the field of pharmaceutical preparations, can solve problems such as small volume limitation, and achieve the effects of high drug loading, mild reaction conditions and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
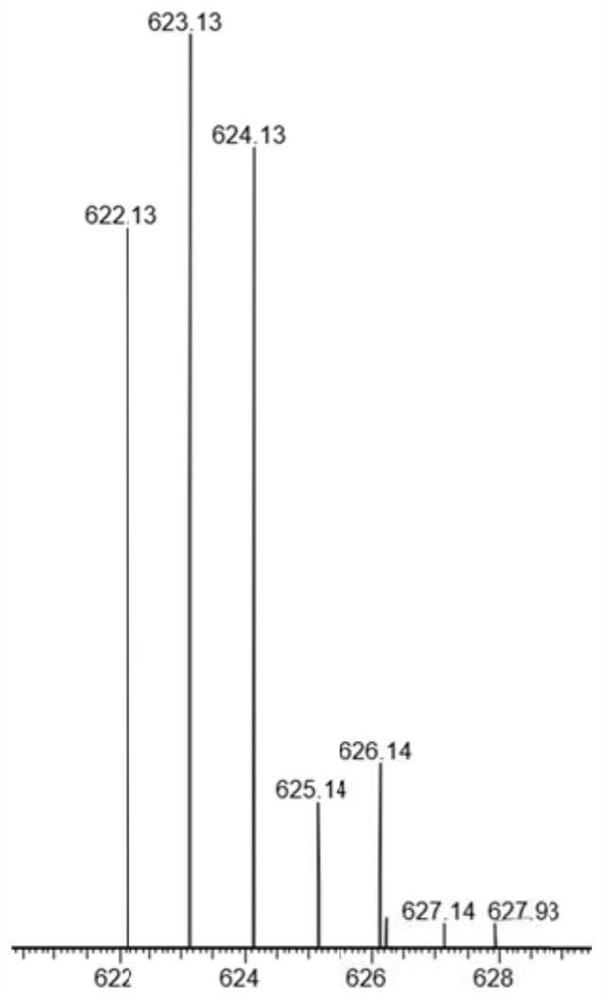
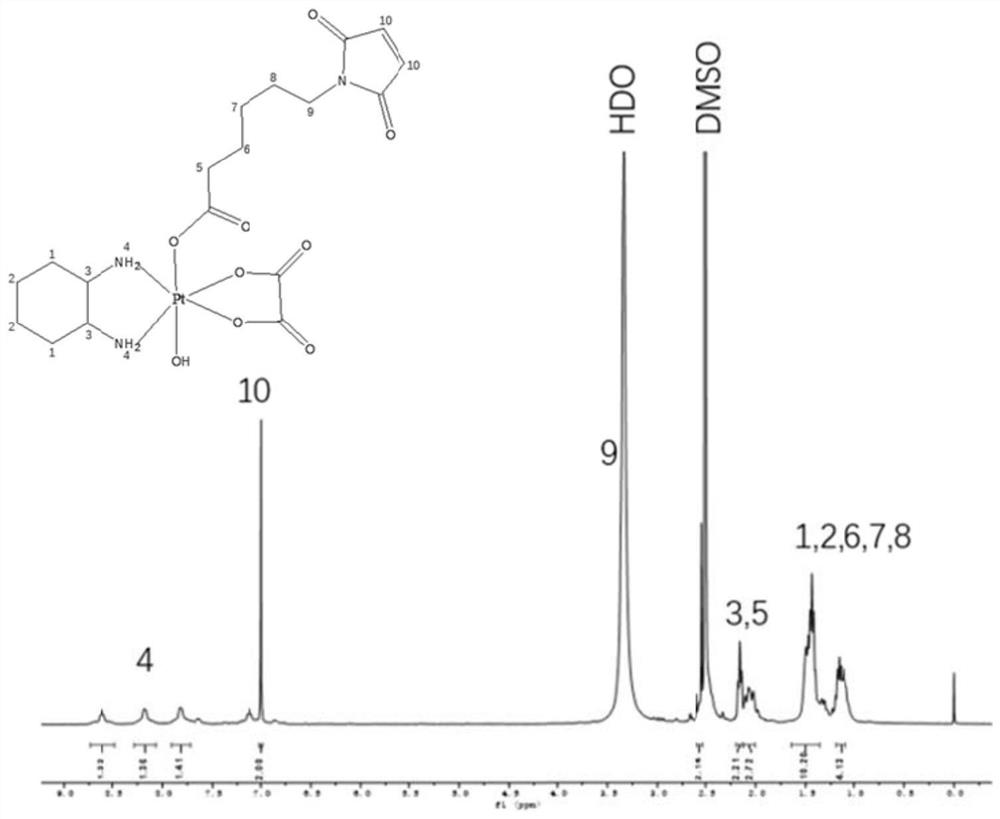
[0067] Embodiment 1: Preparation of tetravalent platinum prodrug
[0068] According to the present invention, we specifically provide the preparation steps of the tetravalent oxaliplatin prodrug in the examples.
[0069] Disperse 300mg oxaliplatin (Shandong Boyuan Pharmaceutical Co., Ltd.) in 12mL 30% H 2 o 2 reaction at 75° C. in the dark for 2 h to obtain a light yellow solution, and the solvent was evaporated on a rotary evaporator. The product was then precipitated three times with a small amount of acetone and a large amount of cold diethyl ether. Vacuum drying gave a light yellow hydroxyoxaliplatin complex (compound 1).
[0070] In order to prepare the tetravalent platinum prodrug of maleimide group functionalization, compound 1 and 6-(maleimide group) hexanoic acid succinimide ester were mixed in a certain ratio of substances (1:0.9) Dissolved in 5 mL of anhydrous DMF, stirred at 50 °C in the dark for 16 h to obtain a yellow solution, and evaporated the solvent on a...
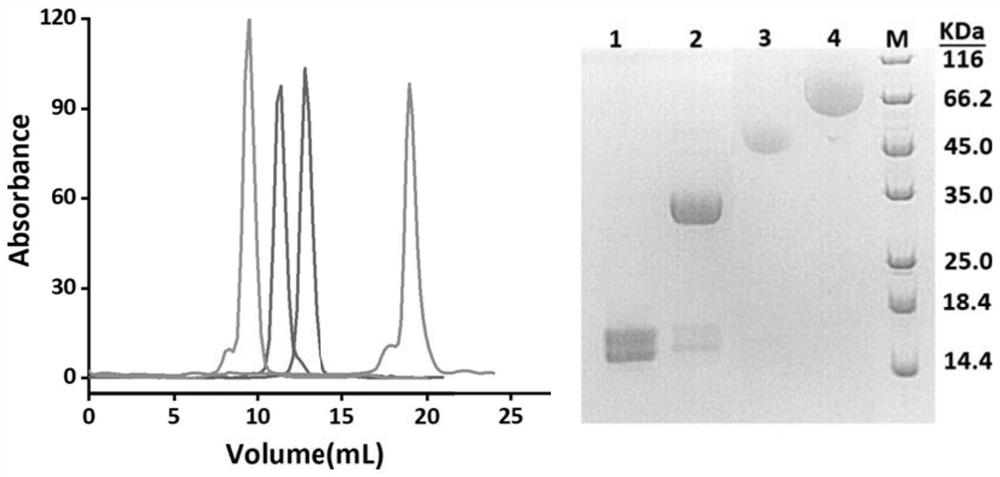
Embodiment 2
[0072] Example 2: Transformation of plasmids targeting EGFR-targeting single-domain antibodies
[0073] According to the method provided by the present invention, the single domain antibody used in this example is an anti-EGFR single domain antibody named 7D12. First, using the polymerase chain reaction (PCR), add "-linker 1-C tag-linker 2-Q tag" to the C-terminus of the 7D12 cDNA sequence synthesized by the whole gene (Sangon Bioengineering Co., Ltd.), and named it 7D12 -C 3 Q. Where connector 1 is 3 (G 4S) (glycine-glycine-glycine-glycine-serine), linker 2 is 3 (GS) (glycine-serine), C tag is C-C-C (cysteine-cysteine-cysteine), Q The label is L-L-Q-S (leucine-leucine-glutamine-serine). After the PCR reaction, the target sequence fragments were recovered by agarose gel electrophoresis.
[0074] Secondly, the recovered target sequence fragments were digested with restriction endonucleases NdeI and XhoI. At the same time, the expression vector pET-22b was digested with th...
Embodiment 3
[0080] Example 3: The modified single domain antibody 7D12-C in Example 2 3 Expression and purification of Q
[0081] 1. 7D12-C 3 Q overexpression in E. coli:
[0082] Transfer the correctly sequenced plasmid obtained in Example 2 into the Rosetta-gami prokaryotic expression strain, spread the bacterial solution, pick a single clone, and expand the culture to 200mL-1000mL LB medium (containing 100 μg / mL ampicillin antibiotic) cultivated in. Strains were grown to OD 600 When =0.8-1.0, add isopropylthiogalactoside (0.2-1mM) and induce at 25°C for 10-15h. After the cultivation, the bacterial cells were collected by centrifugation at 4000 rpm for 20 min at 4°C.
[0083] 2. Use Ni-NTA affinity chromatography resin for 7D12-C 3 Q preliminary purification:
[0084] Resuspend the cells (cells obtained in 20 mL buffer / L cell solution) with binding buffer (50 mM Tris-HCl pH 8.0, 200 mM NaCl). The resuspended bacteria were ultrasonically disrupted (20KHz, 15min). The cell lysate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com