Pharmaceutically acceptable salts of sepiapterin
A mopterin, pharmaceutical technology, applied in the salt and/or co-crystal of mopterin, in the field of treating tetrahydrobiopterin-related diseases, and can solve the problems of neuromotor defects, reducing the formation of neurotransmitters, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0219] Embodiment 1. Preparation of the salt of mepterin
[0220] Production of mepterin and hydrochloric acid, methanesulfonic acid, toluenesulfonic acid, benzenesulfonic acid by slurrying the free base and acid of meptterin in acetone / water (9 / 1, v / v) or methanol for 2-17 days Salts and / or co-crystals of nicotinic acid, niacin, sulfuric acid, phosphoric acid, malonic acid, L-tartaric acid, fumaric acid, gentisic acid and glycolic acid.
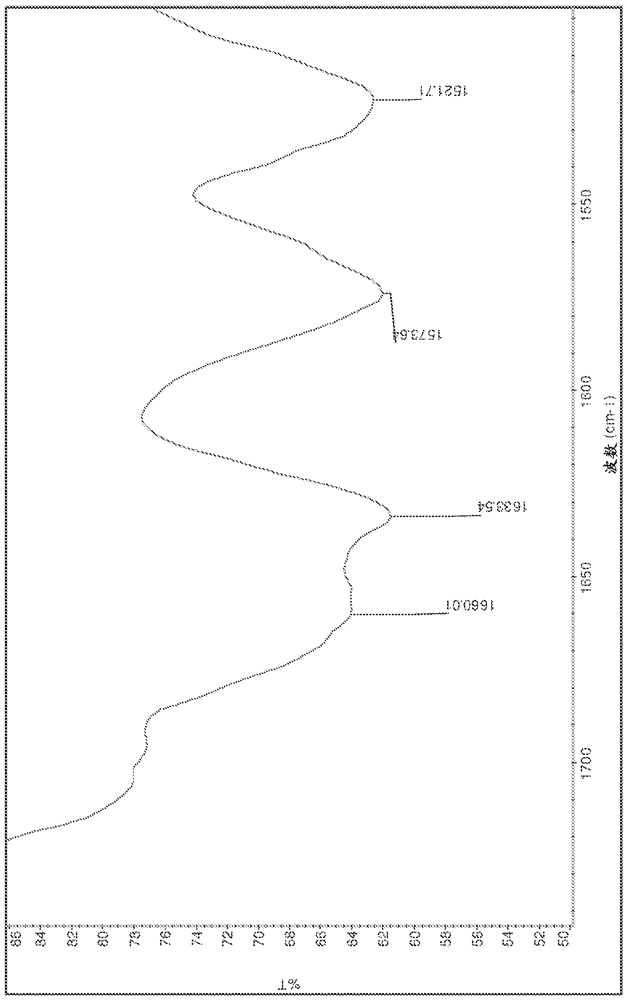
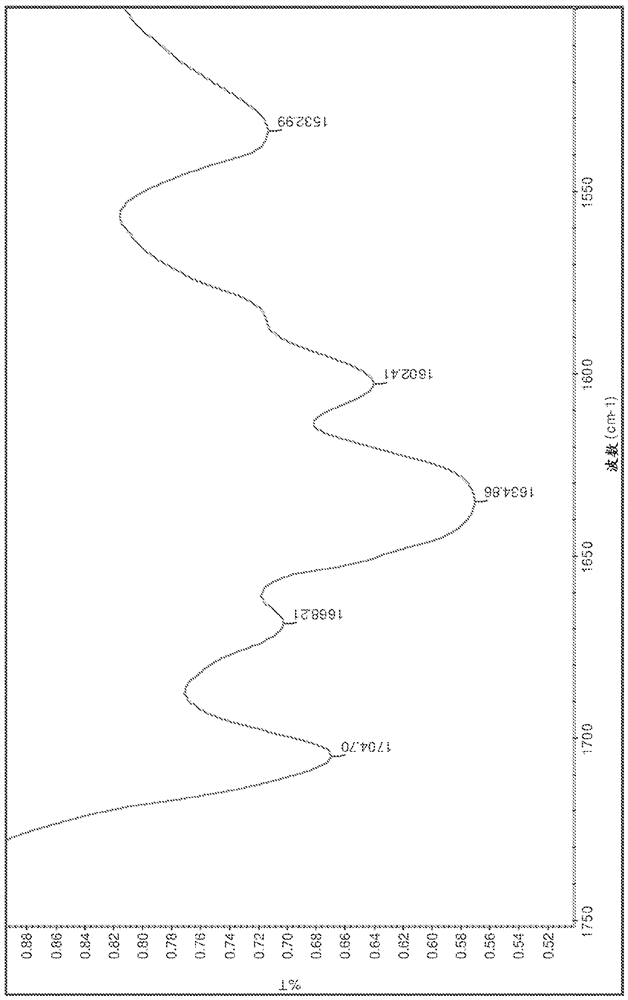
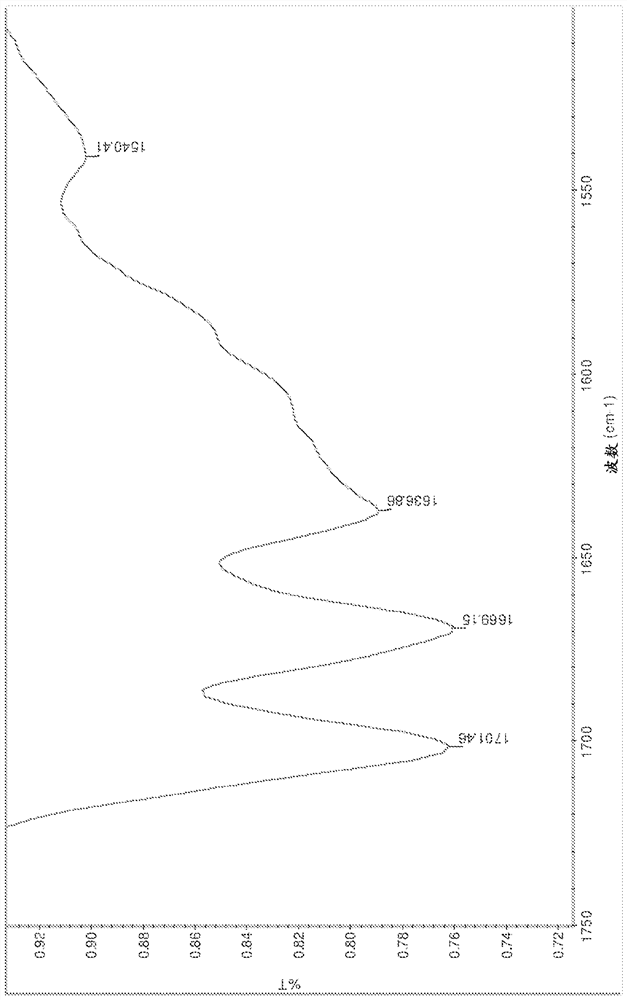
[0221] Salts were analyzed by DSC, TGA, HPLC, IR and XRPD. The results are summarized in Table 15 below. The IR spectrum is displayed on Figure 1-13 middle.
[0222] Table 15. Summary of Mepterin Salts and / or Cocrystal Analysis
[0223]
Embodiment 2
[0224] Example 2. Stability Analysis of Salts and / or Co-crystals of Mepterin
[0225] The prepared salts and / or co-crystals were analyzed for stability after 1 and 2 weeks at 25°C and 60% relative humidity and at 40°C and 75% relative humidity. The results are summarized in Table 16 below. Surprisingly, of all salts and / or co-crystals tested, phosphate and / or co-crystal, tartrate and / or co-crystal and nicotinate and / or co-crystal were significantly more stable than the others. None of the phosphate, tartrate, or nicotinate salts and / or co-crystals underwent a change in form during stability testing, and each of them remained greater than 97% pure over the two weeks studied. In fact, both tartrate and nicotinate remained greater than 99% pure.
[0226] Table 16. Summary of Stability Study Results
[0227]
[0228]
Embodiment 3
[0229] Example 3. Solubility and disproportionation of various mepterin salts and / or co-crystals
[0230] The kinetic solubility of the nicotinate, phosphate, L-tartrate and fumarate salts and / or co-crystals of metopterin was evaluated in water and Medisca Oral Mix. X-ray powder diffraction (XRPD) was performed on the residual solid to identify form change / disproportionation. Suspend the solid in the medium at a target concentration of approximately 7 mg / mL (calculated as free base). The suspension was stirred for 1, 4 and 24 hours at 25 rpm on a rolling incubator. At each time point, 1 mL of the suspension was removed for centrifugation at 10000 rpm (2 min) and filtered through a 0.45 μm membrane to obtain supernatants for HPLC solubility and pH testing, residual solids were analyzed by XRPD. Solubility results are summarized in Tables 17-20.
[0231] Table 17. Solubility Summary of Niacinates and / or Co-crystals
[0232]
[0233] *: Calculated using free base.
[0234...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com