Preparation method of chlorantraniliprole
A technology of chlorantraniliprole and chlorpyridine, which is applied in the field of preparation of chlorantraniliprole, can solve the problems of tedious synthesis steps and low yield of chlorantraniliprole
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A preparation method of chlorantraniliprole, the synthesis reaction of chlorantraniliprole is shown in formula (i), specifically comprising the following steps.
[0035]
[0036] Step 1, the synthesis of 3,6-dichloro-2-hydrazinopyridine:
[0037] Add 2,3,6-trichloropyridine (60g, 0.33mol) and 80% hydrazine hydrate solution (96.6g, 1.55mol) successively into a 500mL four-neck flask equipped with a reflux tube, and add the mixture under stirring at room temperature Catalyst A, catalyst A can be copper chloride, cuprous chloride, tetrabutyl ammonium chloride, benzyl trimethyl ammonium chloride, tetramethyl ammonium chloride, triphenyl butyl phosphorus bromide, triethyl ammonium chloride One or more of methyl ammonium chloride, tetramethyl ammonium bromide, benzyl triethyl ammonium chloride, tetrabutyl ammonium iodide. Catalyst A in this example is tetrabutylammonium chloride (1.8g, 0.006mol). After adding catalyst A, the temperature is raised to 108-110°C, and the refl...
Embodiment 2
[0065] A preparation method of chlorantraniliprole, the synthesis reaction of chlorantraniliprole is shown in formula (i), specifically comprising the following steps.
[0066] Step 1, the synthesis of 3,6-dichloro-2-hydrazinopyridine.
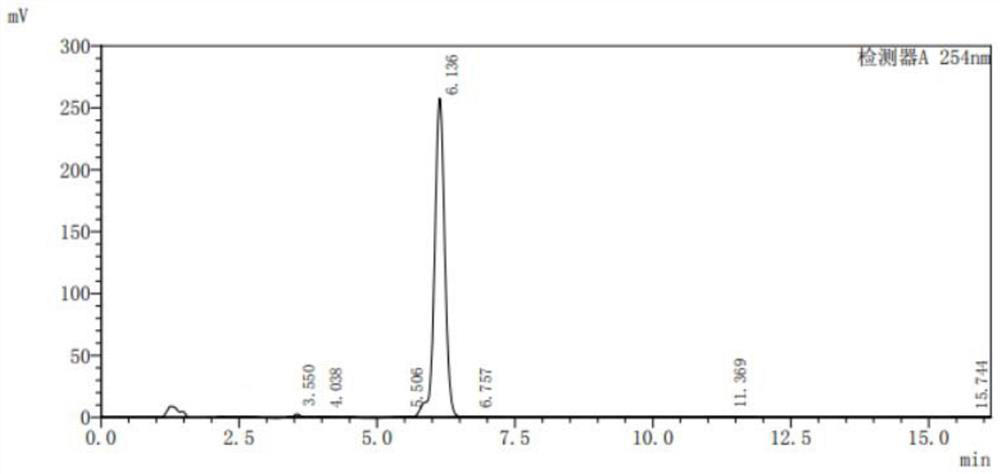
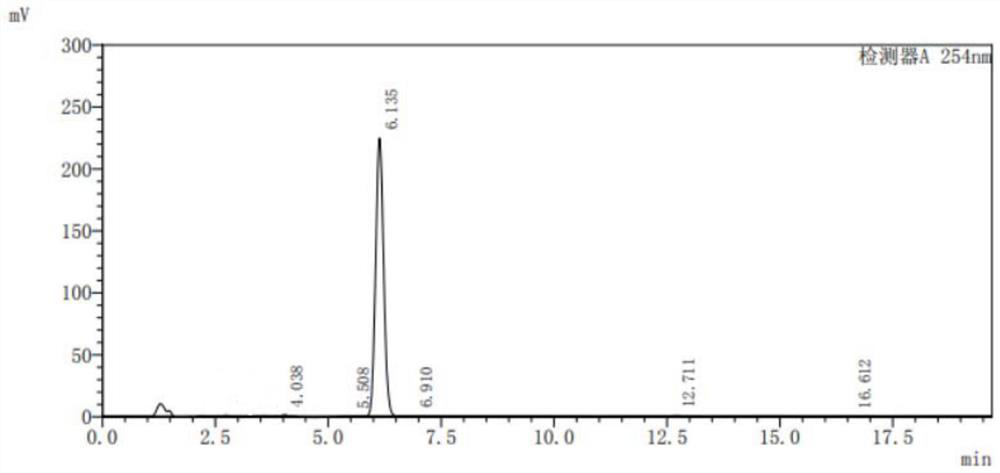
[0067] The difference between this step and step one in Example 1 is: in this step, the addition amount of 80% hydrazine hydrate solution is 90.1g (1.44mol), and catalyst A is cuprous chloride (0.6g, 0.006mol) , reflux reaction for 4 hours, the filter cake was washed with clear water to neutrality and then washed twice with ethanol, and the product 3,6-dichloro-2-hydrazinopyridine was obtained after drying 55.3g, and the HPLC normalized content was 98% (analysis conditions : The chromatographic column is a C18 silica gel reverse-phase column 4.6x150mm 5 μm, the mobile phase is acetonitrile:water:acetic acid=1:1:1%), and the yield is 92.3%.
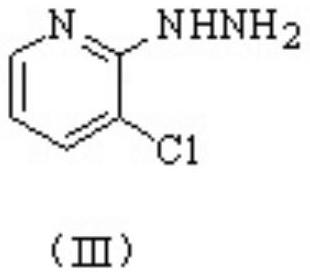
[0068] Step 2, the synthesis of 3-chloro-2-hydrazinopyridine.
[0069] The difference between this st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com