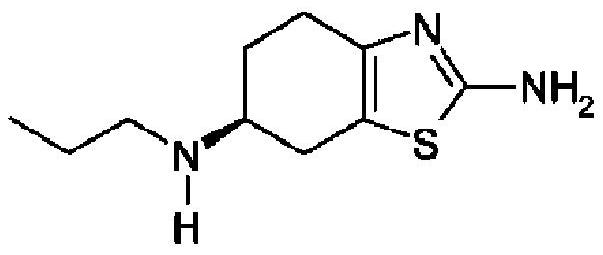
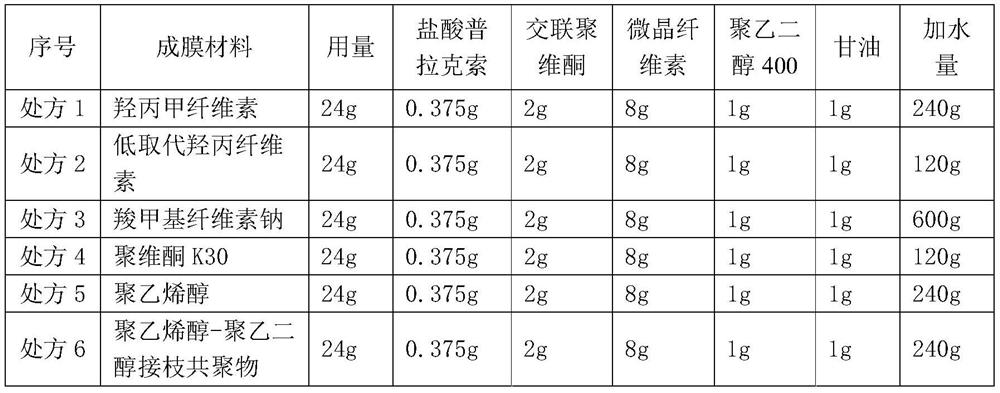
Pramipexole dihydrochloride pharmaceutical composition and preparation method thereof
A technology of pramipexole hydrochloride and copolymer, which is applied in the field of pramipexole hydrochloride orally dissolving film pharmaceutical composition and its preparation, can solve the strict requirements of packaging, storage and transportation, complex preparation process of orally disintegrating tablets, Solve the problems of low tablet hardness, achieve the effect of low cost, good for personnel health protection, and not easy to be brittle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Prescription composition (make 1000 tablets)
[0082] Composition of raw materials Dosage (g) pramipexole hydrochloride 0.1 polyvinyl alcohol 12.77 Crospovidone 3 polyethylene glycol 400 2 glycerin 1 Sucralose 0.1 orange flavor 1 sunset yellow 0.03 Unit film weight (total) 20
[0083] Preparation Process
[0084] (1) Heat polyvinyl alcohol in 100-130 g of purified water to 75-85° C., stir to dissolve, leave to cool to room temperature to replenish volatile water, and obtain solution I;
[0085] (2) Dissolving pramipexole hydrochloride, polyethylene glycol 400, glycerin, sucralose, and orange essence in solution I to obtain solution II;
[0086] (3) Add crospovidone and sunset yellow to solution II and stir to mix evenly to obtain matrix solution III, and vacuumize -0.05Mpa—-0.08Mpa to remove air bubbles for more than 20 minutes, and control the solid content to 15-20 %;
[0087] (4) Apply matrix sol...
Embodiment 2
[0090] Prescription composition (make 1000 tablets)
[0091] Composition of raw materials Dosage (g) pramipexole hydrochloride 0.25 Polyvinyl alcohol-polyethylene glycol graft copolymer 16 Crospovidone 4 microcrystalline cellulose 13 polyethylene glycol 400 3 glycerin 2 Sucralose 0.11 orange flavor 1.6 sunset yellow 0.04 Unit film weight (total) 40
[0092] Preparation Process
[0093] (1) Heat the polyvinyl alcohol-polyethylene glycol graft copolymer in 160-200g of purified water to 75-85°C, stir to dissolve, leave to cool to room temperature to replenish the volatile water, and obtain solution I;
[0094] (2) Dissolving pramipexole hydrochloride, polyethylene glycol 400, glycerin, sucralose, and orange flavor in solution I to obtain solution II;
[0095] (3) Add crospovidone, microcrystalline cellulose, and sunset yellow into solution II and stir and mix evenly to obtain matrix liquid III, and remove...
Embodiment 3
[0099] Prescription composition (make 1000 tablets)
[0100]
[0101]
[0102] Preparation Process
[0103] (1) Heat polyvinyl alcohol in 200-240g of purified water to 75-85°C, stir to dissolve, leave to cool to room temperature to supplement the volatilized water, and obtain solution I;
[0104] (2) Dissolving pramipexole hydrochloride, polyethylene glycol 400, glycerin, sucralose, and orange flavor in solution I to obtain solution II;
[0105] (3) Add crospovidone, microcrystalline cellulose, and sunset yellow into solution II and stir and mix evenly to obtain matrix liquid III, and remove air bubbles for more than 20 minutes under vacuum-0.05Mpa—-0.08Mpa conditions, and control solid The content is 25-30%;
[0106] (4) Coat the matrix solution III with a coating machine, control the coating wet thickness to 200-250um, and dry and roll at 60-75°C;
[0107] (5) Cut the winding film, the cutting width of the film is: 2×4cm 2 , the control stresses the difference ± 6%, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com