Synthesis method of 1, 3-diaryl substituted tetrazolone inner salt
A synthesis method and technology of tetrazolone, applied in the field of organic chemical synthesis, can solve the problems of low yield and the like, and achieve the effects of high yield, few by-products and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The synthetic method comprises the following steps:
[0031] a. In the air atmosphere, after mixing 0.5mmol of diaryl semicarbazide with 10mol% catalyst palladium acetate, and adding 1.0mmol of potassium carbonate as alkali, the above mixed solution is placed in a solvent, and the reaction temperature is controlled at 70- Stir by magnetic force for 2-4 hours at 100°C;
[0032] b. During the reaction process, use TLC to monitor until the reaction is complete. After the reaction, cool down to room temperature. After the reaction, add an appropriate amount of water to quench the reaction, then add an appropriate amount of dichloromethane for extraction, dry over anhydrous sodium sulfate, and spin dry under reduced pressure The pure 1,3-diaryl substituted tetrazolone internal salt was separated by column chromatography.
[0033] In this embodiment, it is any one of dimethyl sulfoxide, tetrahydrofuran, and dioxane. The solvent is preferably dimethylsulfoxide.
[0034] In ...
Embodiment 1
[0040]
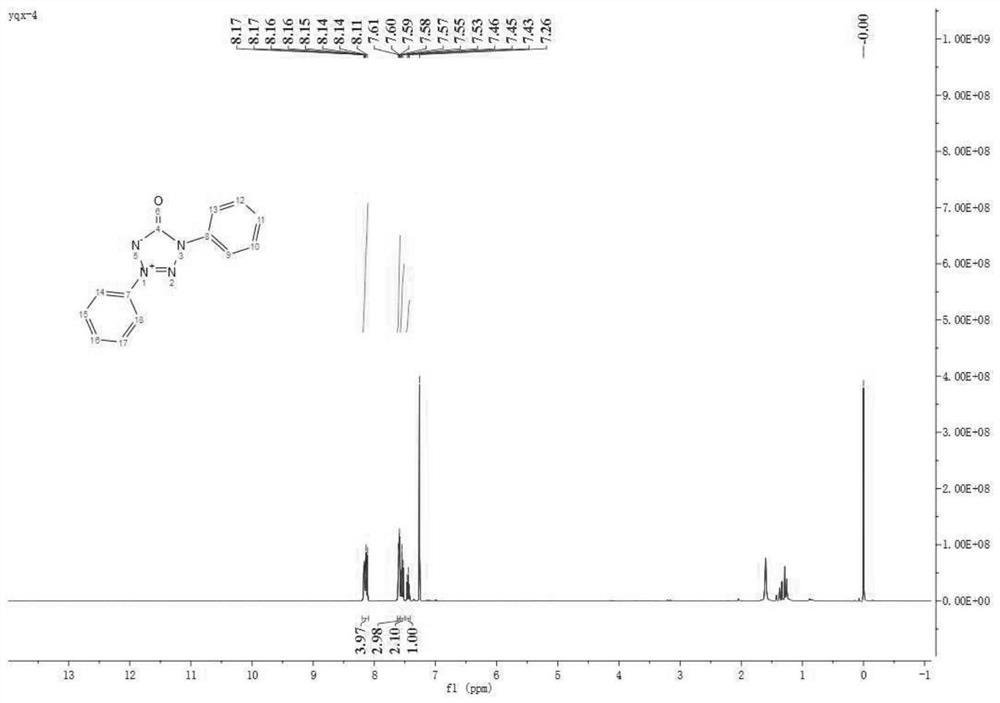
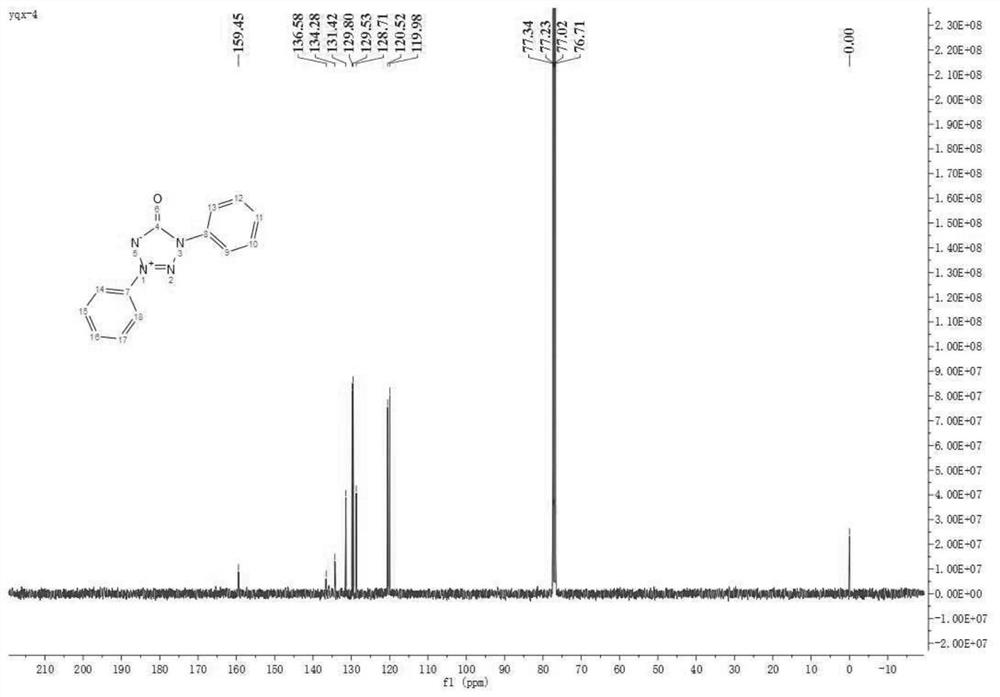
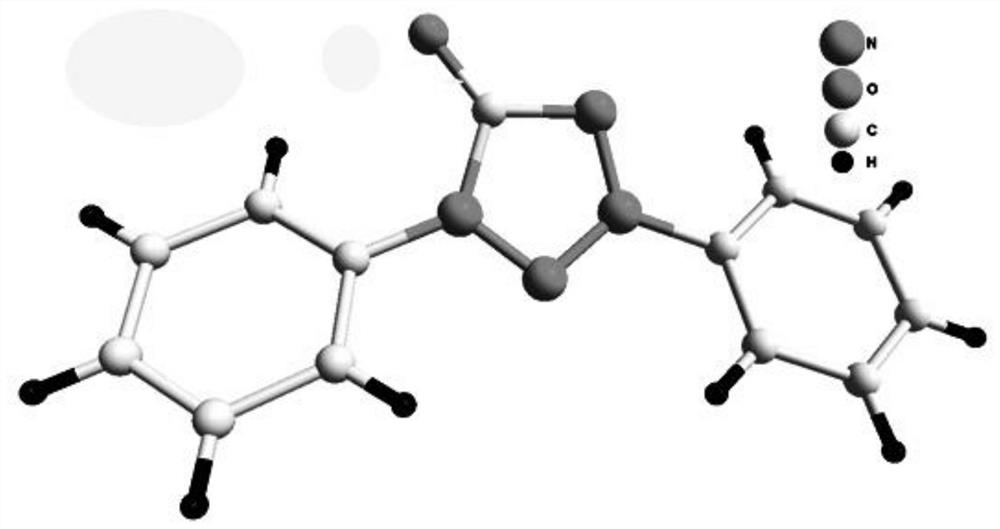
[0041] In air atmosphere, 0.5 mmol of diaryl semicarbazide, 10 mol% of palladium acetate, and 1.0 mmol of potassium carbonate were magnetically stirred in DMSO at 90° C. for 3 h, and the reaction was monitored by TLC until complete reaction. Add an appropriate amount of water to quench the reaction in the post-treatment, then add an appropriate amount of dichloromethane to extract, dry over anhydrous sodium sulfate, spin the solvent under reduced pressure, and then separate the pure 1,3-diphenyltetrazolone internal salt 2a by column chromatography . Isolated yield: 84%. The structure of the compound has been determined by single crystal X-ray diffraction, CCDC number: 1919662.
[0042] 1 H NMR (400MHz, CDCl 3 )δ8.17-8.11 (m, 4H), 7.59 (dd, J = 6.8, 4.0Hz, 3H), 7.55 (t, J = 7.8Hz, 2H), 7.45 (t, J = 7.4Hz, 1H); 13 C NMR (101MHz, CDCl 3 )δ159.45, 136.58, 134.28, 131.42, 129.80, 129.53, 128.71, 120.52, 119.98; HRMS (ESI-TOF) calcd.For C 13 h 10 N 4 NaO + :261....
Embodiment 2
[0044] In an air atmosphere, 0.5 mmol of diaryl semicarbazide, 10 mol% of palladium acetate, and 1.0 mmol of potassium carbonate were magnetically stirred in tetrahydrofuran at 80° C. for 3 h, and the reaction was monitored by TLC until complete reaction. Add an appropriate amount of water to quench the reaction in the post-treatment, then add an appropriate amount of dichloromethane to extract, dry over anhydrous sodium sulfate, spin the solvent under reduced pressure, and then separate the pure 1,3-diphenyltetrazolone internal salt 2a by column chromatography . Isolated yield: 58%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com