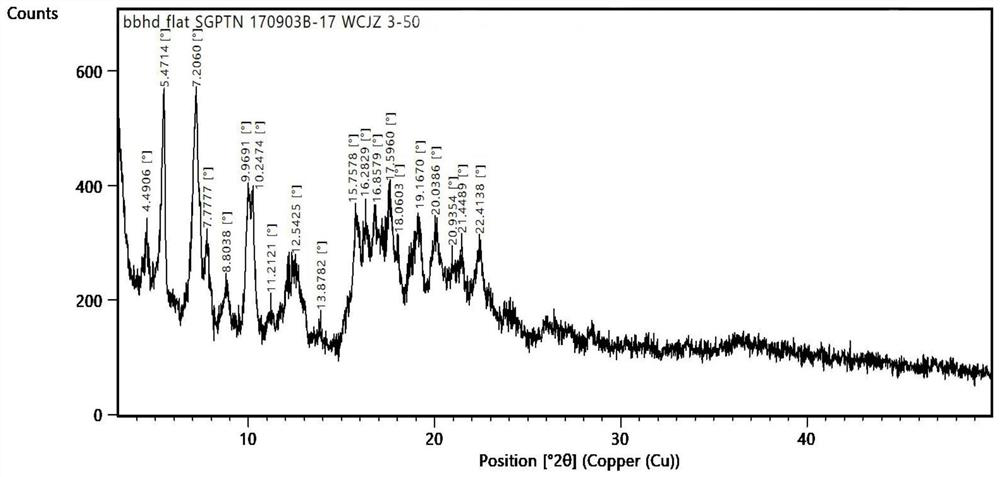
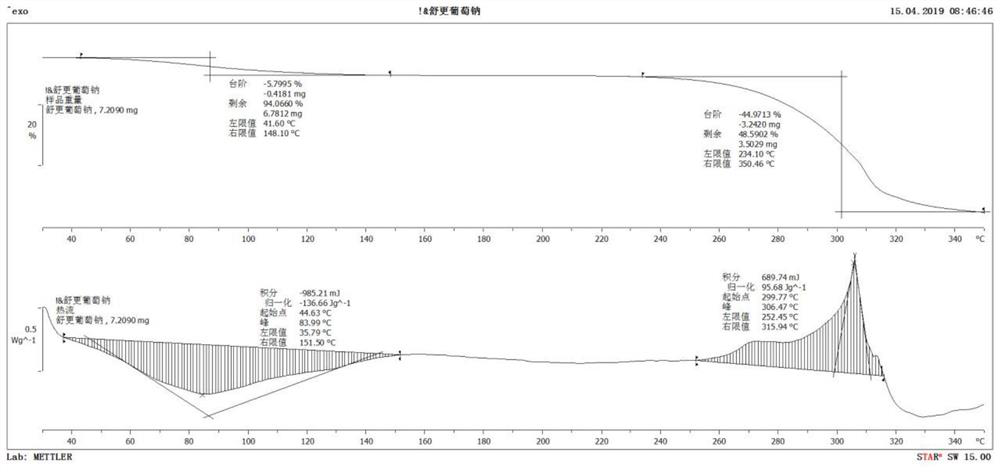
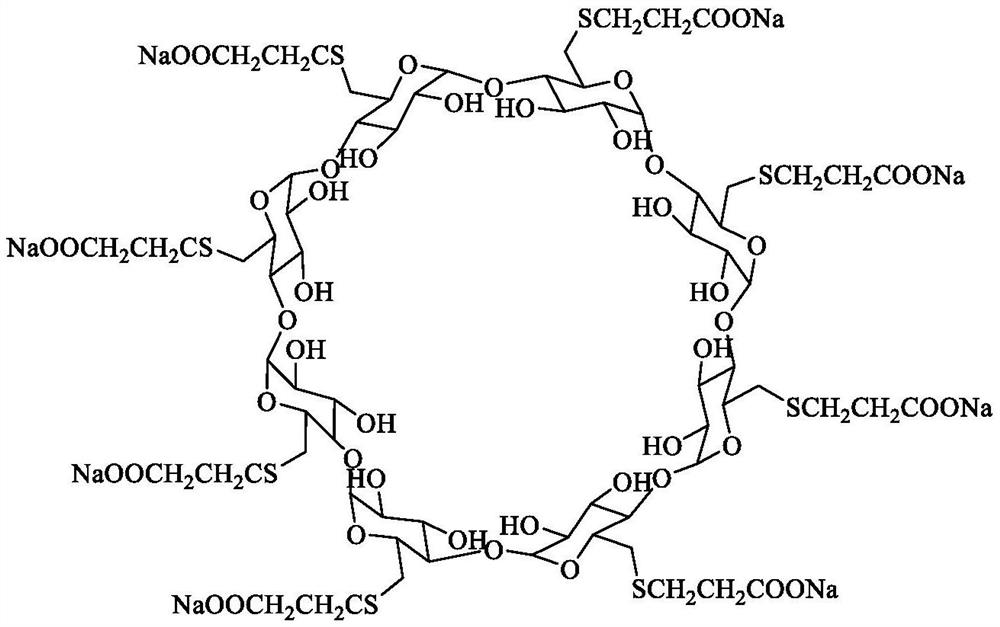
Sugammadex sodium crystal form
A technology of sugammadex sodium and crystal form, applied in the field of crystal form drug molecules, can solve the problems of thermal stability, photostability, dissolution, and bioavailability that cannot be well satisfied, and is suitable for large-scale promotion. Application, qualified solution clarity, beneficial effect on drug storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Add sugammadex sodium (20.05g) into purified water (100ml) and stir to dissolve; slowly add ethanol (20ml) dropwise to the sugammadex sodium aqueous solution, put it into a lyophilizer, and maintain it at -40°C for 12 hours ; After it is completely solidified, open the cold trap and vacuumize. When the vacuum reaches 150mT, raise the temperature to -20°C at 1°C / 4min, and maintain at -20°C for 15 hours for sublimation drying. ℃ / 2min to raise the temperature to 5°C, and maintain at 5°C for 15 hours for analysis and drying. After drying, close the cold trap to obtain white crystals of sugammadex sodium with a purity of 99.85%.
Embodiment 2
[0054] Add sugammadex sodium (20.03g) into purified water (90ml) and stir to dissolve; slowly add acetone (10ml) dropwise to sugammadex sodium aqueous solution, put it into a lyophilizer, and maintain it at -30°C for 15 hours ; After it is completely solidified, open the cold trap and vacuumize. When the vacuum reaches 150mT, raise the temperature to -15°C at 1°C / 3min, and maintain at -15°C for 20 hours for sublimation drying. ℃ / 3min to raise the temperature to 0°C, and maintain at 0°C for 20 hours for analysis and drying. After drying, close the cold trap to obtain white crystalline sugammadex sodium with a purity of 99.81%.
Embodiment 3
[0056] Add sugammadex sodium (20.06g) into purified water (100ml) and stir to dissolve; slowly add methanol (30ml) dropwise to sugammadex sodium aqueous solution, put it into a lyophilizer, and maintain it at -45°C for 14 hours ; After it is completely solidified, open the cold trap and vacuumize. When the vacuum reaches 150mT, raise the temperature to -25°C at 1°C / 4min, and maintain at -25°C for 20 hours for sublimation drying. ℃ / 2min to raise the temperature to 10°C, and maintain at 10°C for 15 hours for analysis and drying. After drying, close the cold trap to obtain white crystalline sugammadex sodium with a purity of 99.80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com